Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-12-01 , DOI: 10.1016/j.cej.2022.140671 Yanbin Wang , Haibo Shen , Zezhou Shi , Qiushuang Xing , Yunqing Pi
|
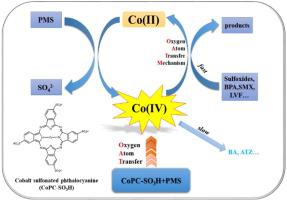
Sulfate radicals (SO4•-) are typically regarded to be the dominant active species in the cobalt-activated peroxymonosulfate (PMS) processes. In this study, a water-soluble sulfonated cobalt phthalocyanine (CoPcS) was applied as a molecular catalyst to activate PMS and break down organic contaminants. The CoPcS/PMS system was shown to be dominated by high-valent cobalt-oxo species (Co(IV)) rather than the conventional SO–4• and hydroxyl radical (•OH) based on methyl phenyl sulfoxide (PMSO) chemical probe, radical quenching experiments, and electron paramagnetic resonance (EPR). The nonradical oxidation process was found to be predominant in acid conditions (pH 3-5), whereas the radical oxidation pathway co-occurred in neutral or alkaline medium conditions (pH 7-8). Organic contaminants with low ionization potential (IP) can be effectively and selectively removed by the CoPcS/PMS system. More notably, the removal of organic pollutants in the CoPcS/PMS system was not significantly impacted by the ubiquitous inorganic anions (such as SO42-, HCO3-, H2PO4- and HPO42-) or dissolved organic matter (e.g., humic acid). The promoting effect for the removal of levofloxacin was observed under the high concentration of HPO42- conditions. The CoPcS/PMS system demonstrated resistance to the environmental matrix and maintained its oxidation capacity at the practical aqueous substrates (tap water and secondary effluent).











































 京公网安备 11010802027423号
京公网安备 11010802027423号