当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfite activation for ciprofloxacin rapid degradation using an iron-based metal organic framework derivative in heterogeneous processes: Performance and mechanisms investigation
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-11-30 , DOI: 10.1016/j.cej.2022.140644 Mingming Wang , Xue Huang , Benyin Zhang , Shijin Zhang , Jing Zhang , Qingguo Wang
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-11-30 , DOI: 10.1016/j.cej.2022.140644 Mingming Wang , Xue Huang , Benyin Zhang , Shijin Zhang , Jing Zhang , Qingguo Wang
|
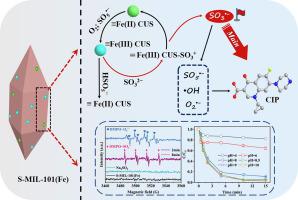
Owing to its low eco-toxicity and low cost, sulfite is considered a promising precursor for oxysulfur radicals. In this study, we report the rapid degradation of ciprofloxacin in water using a derivative of an iron-based metal–organic framework catalyst (S-MIL-101(Fe)) for the heterogeneous activation of sulfite. S-MIL-101(Fe) possessed a similar structure to the original MIL-101(Fe), and more active sites were exposed. Unlike previous systems used to activate sulfite with Fe-based catalysts, this system exhibited excellent performance under alkaline conditions. Ciprofloxacin (10 mg/L) removal efficiency of 94.7 % was observed at pH 8.7, implying a different activation mechanism. It is suggested that the iron coordinatively unsaturated metal sites (Fe CUSs) on the surface of S-MIL-101(Fe) can effectively complex with SO to form Fe(III) CUS-SO, followed by the generation of SO through single-electron transfer. Quenching and electron paramagnetic resonance experiments demonstrated that SO, O, SO and OH were involved in the degradation of ciprofloxacin, in which SO played a significant role. Moreover, HSO (peroxymonosulfate ion), an important product produced during the sulfite activation process, also participated in the formation of free radicals. This study complements the mechanism of heterogeneous activation of sulfites by Fe-based materials and reinforces the important role played by SO in some cases. The influence of chloride, bicarbonate, nitrate, and humic acid on ciprofloxacin elimination was minimal. In addition, this system could operate efficiently in real water environments.
中文翻译:

在非均相过程中使用铁基金属有机骨架衍生物进行亚硫酸盐活化以实现环丙沙星快速降解:性能和机制研究
由于其低生态毒性和低成本,亚硫酸盐被认为是氧硫自由基的有前途的前体。在这项研究中,我们报告了使用铁基金属有机骨架催化剂(S-MIL-101(Fe))的衍生物对亚硫酸盐进行非均相活化,可快速降解水中的环丙沙星。 S-MIL-101(Fe)与原始MIL-101(Fe)具有相似的结构,并且暴露出更多的活性位点。与以前使用铁基催化剂活化亚硫酸盐的系统不同,该系统在碱性条件下表现出优异的性能。在 pH 8.7 时观察到环丙沙星 (10 mg/L) 的去除效率为 94.7%,这意味着不同的激活机制。这表明S-MIL-101(Fe)表面的铁配位不饱和金属位点(Fe CUSs)可以有效地与SO络合形成Fe(III) CUS-SO,然后通过单配位生成SO电子转移。猝灭和电子顺磁共振实验表明SO、O、SO和OH参与了环丙沙星的降解,其中SO起重要作用。此外,亚硫酸盐活化过程中产生的重要产物HSO(过一硫酸根离子)也参与了自由基的形成。这项研究补充了铁基材料对亚硫酸盐的非均相活化机制,并强化了SO在某些情况下发挥的重要作用。氯化物、碳酸氢盐、硝酸盐和腐殖酸对环丙沙星消除的影响很小。此外,该系统可以在真实的水环境中高效运行。
更新日期:2022-11-30
中文翻译:

在非均相过程中使用铁基金属有机骨架衍生物进行亚硫酸盐活化以实现环丙沙星快速降解:性能和机制研究
由于其低生态毒性和低成本,亚硫酸盐被认为是氧硫自由基的有前途的前体。在这项研究中,我们报告了使用铁基金属有机骨架催化剂(S-MIL-101(Fe))的衍生物对亚硫酸盐进行非均相活化,可快速降解水中的环丙沙星。 S-MIL-101(Fe)与原始MIL-101(Fe)具有相似的结构,并且暴露出更多的活性位点。与以前使用铁基催化剂活化亚硫酸盐的系统不同,该系统在碱性条件下表现出优异的性能。在 pH 8.7 时观察到环丙沙星 (10 mg/L) 的去除效率为 94.7%,这意味着不同的激活机制。这表明S-MIL-101(Fe)表面的铁配位不饱和金属位点(Fe CUSs)可以有效地与SO络合形成Fe(III) CUS-SO,然后通过单配位生成SO电子转移。猝灭和电子顺磁共振实验表明SO、O、SO和OH参与了环丙沙星的降解,其中SO起重要作用。此外,亚硫酸盐活化过程中产生的重要产物HSO(过一硫酸根离子)也参与了自由基的形成。这项研究补充了铁基材料对亚硫酸盐的非均相活化机制,并强化了SO在某些情况下发挥的重要作用。氯化物、碳酸氢盐、硝酸盐和腐殖酸对环丙沙星消除的影响很小。此外,该系统可以在真实的水环境中高效运行。









































 京公网安备 11010802027423号
京公网安备 11010802027423号