当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gas-Phase Formation of 1,3,5,7-Cyclooctatetraene (C8H8) through Ring Expansion via the Aromatic 1,3,5-Cyclooctatrien-7-yl Radical (C8H9•) Transient
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c06448 Zhenghai Yang 1 , Galiya R Galimova 2 , Chao He 1 , Srinivas Doddipatla 1 , Alexander M Mebel 2 , Ralf I Kaiser 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c06448 Zhenghai Yang 1 , Galiya R Galimova 2 , Chao He 1 , Srinivas Doddipatla 1 , Alexander M Mebel 2 , Ralf I Kaiser 1
Affiliation
|
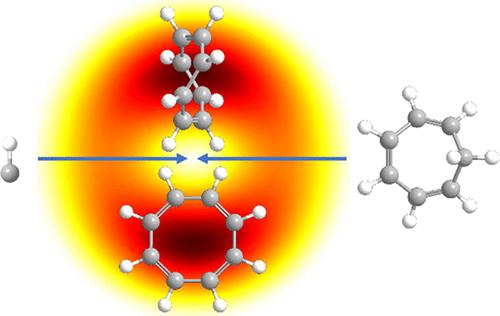
Gas-phase 1,3,5,7-cyclooctatetraene (C8H8) and triplet aromatic 1,3,5,7-cyclooctatetraene (C8H8) were formed for the first time through bimolecular methylidyne radical (CH)–1,3,5-cycloheptatriene (C7H8) reactions under single-collision conditions on a doublet surface. The reaction involves methylidyne radical addition to the olefinic π electron system of 1,3,5-cycloheptatriene followed by isomerization and ring expansion to an aromatic 1,3,5-cyclooctatrien-7-yl radical (C8H9•). The chemically activated doublet radical intermediate undergoes unimolecular decomposition to 1,3,5,7-cyclooctatetraene. Substituted 1,3,5,7-cyclooctatetraene molecules can be prepared in the gas phase with hydrogen atom(s) in the 1,3,5-cycloheptatriene reactant being replaced by organic side groups. These findings are also of potential interest to organometallic chemists by expanding the synthesis of exotic transition-metal complexes incorporating substituted 1,3,5,7-cyclooctatetraene dianion (C8H82–) ligands and to untangle the unimolecular decomposition of chemically activated and substituted 1,3,5-cyclooctatrien-7-yl radical, eventually gaining a fundamental insight of their bonding chemistry, electronic structures, and stabilities.
中文翻译:

1,3,5,7-环辛四烯 (C8H8) 通过芳香族 1,3,5-环辛三烯-7-基自由基 (C8H9•) 瞬态扩环形成气相
首次通过双分子亚甲基自由基 (CH)– 形成气相 1,3,5,7-环辛四烯 (C 8 H 8 ) 和三线态芳香族 1,3,5,7-环辛四烯 (C 8 H 8 )–双峰表面上单碰撞条件下的1,3,5-环庚三烯 (C 7 H 8 ) 反应。该反应涉及亚甲基自由基加成到 1,3,5-环庚三烯的烯属 π 电子系统,然后异构化和扩环为芳香族 1,3,5-环辛三烯-7-基 (C 8 H 9 •). 化学活化的双线态自由基中间体经历单分子分解为 1,3,5,7-环辛四烯。取代的 1,3,5,7-环辛四烯分子可以在气相中制备,其中 1,3,5-环庚三烯反应物中的氢原子被有机侧基取代。这些发现也可能引起有机金属化学家的兴趣,因为他们可以扩展包含取代的 1,3,5,7-环辛四烯双阴离子 (C 8 H 8 2– ) 配体的外来过渡金属配合物的合成,并解开化学活化的单分子分解并取代 1,3,5-cyclooctatrien-7-yl 自由基,最终对它们的键合化学、电子结构和稳定性有了基本的了解。
更新日期:2022-12-01
中文翻译:

1,3,5,7-环辛四烯 (C8H8) 通过芳香族 1,3,5-环辛三烯-7-基自由基 (C8H9•) 瞬态扩环形成气相
首次通过双分子亚甲基自由基 (CH)– 形成气相 1,3,5,7-环辛四烯 (C 8 H 8 ) 和三线态芳香族 1,3,5,7-环辛四烯 (C 8 H 8 )–双峰表面上单碰撞条件下的1,3,5-环庚三烯 (C 7 H 8 ) 反应。该反应涉及亚甲基自由基加成到 1,3,5-环庚三烯的烯属 π 电子系统,然后异构化和扩环为芳香族 1,3,5-环辛三烯-7-基 (C 8 H 9 •). 化学活化的双线态自由基中间体经历单分子分解为 1,3,5,7-环辛四烯。取代的 1,3,5,7-环辛四烯分子可以在气相中制备,其中 1,3,5-环庚三烯反应物中的氢原子被有机侧基取代。这些发现也可能引起有机金属化学家的兴趣,因为他们可以扩展包含取代的 1,3,5,7-环辛四烯双阴离子 (C 8 H 8 2– ) 配体的外来过渡金属配合物的合成,并解开化学活化的单分子分解并取代 1,3,5-cyclooctatrien-7-yl 自由基,最终对它们的键合化学、电子结构和稳定性有了基本的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号