当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical and Photophysical Dynamics of the Aqueous Ferrate(VI) Ion
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c08048 Cali Antolini 1 , Charles D Spellman 2 , Christopher J Otolski 3 , Gilles Doumy 3 , Anne Marie March 3 , Donald A Walko 4 , Cunming Liu 4 , Xiaoyi Zhang 4 , Benjamin T Young 5 , Joseph E Goodwill 2 , Dugan Hayes 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c08048 Cali Antolini 1 , Charles D Spellman 2 , Christopher J Otolski 3 , Gilles Doumy 3 , Anne Marie March 3 , Donald A Walko 4 , Cunming Liu 4 , Xiaoyi Zhang 4 , Benjamin T Young 5 , Joseph E Goodwill 2 , Dugan Hayes 1
Affiliation
|
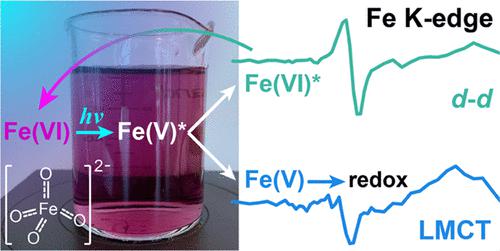
Ferrate(VI) has the potential to play a key role in future water supplies. Its salts have been suggested as “green” alternatives to current advanced oxidation and disinfection methods in water treatment, especially when combined with ultraviolet light to stimulate generation of highly oxidizing Fe(V) and Fe(IV) species. However, the nature of these intermediates, the mechanisms by which they form, and their roles in downstream oxidation reactions remain unclear. Here, we use a combination of optical and X-ray transient absorption spectroscopies to study the formation, interconversion, and relaxation of several excited-state and metastable high-valent iron species following excitation of aqueous potassium ferrate(VI) by ultraviolet and visible light. Branching from the initially populated ligand-to-metal charge transfer state into independent photophysical and photochemical pathways occurs within tens of picoseconds, with the quantum yield for the generation of reactive Fe(V) species determined by relative rates of the competing intersystem crossing and reverse electron transfer processes. Relaxation of the metal-centered states then occurs within 4 ns, while the formation of metastable Fe(V) species occurs in several steps with time constants of 250 ps and 300 ns. Results here improve the mechanistic understanding of the formation and fate of Fe(V) and Fe(IV), which will accelerate the development of novel advanced oxidation processes for water treatment applications.
中文翻译:

高铁酸盐 (VI) 离子水溶液的光化学和光物理动力学
高铁酸盐 (VI) 有可能在未来的供水中发挥关键作用。它的盐类被认为是目前水处理中高级氧化和消毒方法的“绿色”替代品,尤其是当与紫外线结合以刺激高氧化性 Fe(V) 和 Fe(IV) 物质的产生时。然而,这些中间体的性质、它们形成的机制以及它们在下游氧化反应中的作用仍不清楚。在这里,我们使用光学和 X 射线瞬态吸收光谱学的组合来研究在紫外和可见光激发高铁酸钾 (VI) 水溶液后几种激发态和亚稳态高价铁物质的形成、相互转化和弛豫. 从最初填充的配体到金属电荷转移状态分支到独立的光物理和光化学途径发生在几十皮秒内,产生反应性 Fe(V) 物种的量子产率由竞争的系统间交叉和反向的相对速率决定电子转移过程。然后,以金属为中心的状态的弛豫在 4 ns 内发生,而亚稳态 Fe(V) 物种的形成分几个步骤发生,时间常数为 250 ps 和 300 ns。这里的结果提高了对 Fe(V) 和 Fe(IV) 的形成和归宿的机理理解,这将加速用于水处理应用的新型高级氧化工艺的开发。产生反应性 Fe(V) 物种的量子产率由竞争性系统间交叉和反向电子转移过程的相对速率决定。然后,以金属为中心的状态的弛豫在 4 ns 内发生,而亚稳态 Fe(V) 物种的形成分几个步骤发生,时间常数为 250 ps 和 300 ns。这里的结果提高了对 Fe(V) 和 Fe(IV) 的形成和归宿的机理理解,这将加速用于水处理应用的新型高级氧化工艺的开发。产生反应性 Fe(V) 物种的量子产率由竞争性系统间交叉和反向电子转移过程的相对速率决定。然后,以金属为中心的状态的弛豫在 4 ns 内发生,而亚稳态 Fe(V) 物种的形成分几个步骤发生,时间常数为 250 ps 和 300 ns。这里的结果提高了对 Fe(V) 和 Fe(IV) 的形成和归宿的机理理解,这将加速用于水处理应用的新型高级氧化工艺的开发。
更新日期:2022-12-01
中文翻译:

高铁酸盐 (VI) 离子水溶液的光化学和光物理动力学
高铁酸盐 (VI) 有可能在未来的供水中发挥关键作用。它的盐类被认为是目前水处理中高级氧化和消毒方法的“绿色”替代品,尤其是当与紫外线结合以刺激高氧化性 Fe(V) 和 Fe(IV) 物质的产生时。然而,这些中间体的性质、它们形成的机制以及它们在下游氧化反应中的作用仍不清楚。在这里,我们使用光学和 X 射线瞬态吸收光谱学的组合来研究在紫外和可见光激发高铁酸钾 (VI) 水溶液后几种激发态和亚稳态高价铁物质的形成、相互转化和弛豫. 从最初填充的配体到金属电荷转移状态分支到独立的光物理和光化学途径发生在几十皮秒内,产生反应性 Fe(V) 物种的量子产率由竞争的系统间交叉和反向的相对速率决定电子转移过程。然后,以金属为中心的状态的弛豫在 4 ns 内发生,而亚稳态 Fe(V) 物种的形成分几个步骤发生,时间常数为 250 ps 和 300 ns。这里的结果提高了对 Fe(V) 和 Fe(IV) 的形成和归宿的机理理解,这将加速用于水处理应用的新型高级氧化工艺的开发。产生反应性 Fe(V) 物种的量子产率由竞争性系统间交叉和反向电子转移过程的相对速率决定。然后,以金属为中心的状态的弛豫在 4 ns 内发生,而亚稳态 Fe(V) 物种的形成分几个步骤发生,时间常数为 250 ps 和 300 ns。这里的结果提高了对 Fe(V) 和 Fe(IV) 的形成和归宿的机理理解,这将加速用于水处理应用的新型高级氧化工艺的开发。产生反应性 Fe(V) 物种的量子产率由竞争性系统间交叉和反向电子转移过程的相对速率决定。然后,以金属为中心的状态的弛豫在 4 ns 内发生,而亚稳态 Fe(V) 物种的形成分几个步骤发生,时间常数为 250 ps 和 300 ns。这里的结果提高了对 Fe(V) 和 Fe(IV) 的形成和归宿的机理理解,这将加速用于水处理应用的新型高级氧化工艺的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号