当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-Catalyzed Anti-Markovnikov Hydroamidation of Unactivated Alkenes Using Dioxazolones as Amidating Reagents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c10552 Noah Wagner-Carlberg 1 , Tomislav Rovis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-12-01 , DOI: 10.1021/jacs.2c10552 Noah Wagner-Carlberg 1 , Tomislav Rovis 1
Affiliation
|
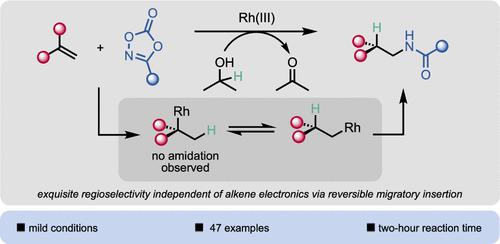
The amide is one of the most prevalent functional groups in all of pharmaceuticals, and for this reason, reactions that introduce the amide moiety are of particular value. Intermolecular hydroamidation of alkenes remains an underexplored method for the synthesis of amide-containing compounds. The majority of hydroamidation procedures exhibit Markovnikov regioselectivity, while current methods for anti-Markovnikov hydroamidation are somewhat limited to activated alkene substrates or radical processes. Herein, we report a general method for the intermolecular anti-Markovnikov hydroamidation of unactivated alkenes under mild conditions, utilizing Rh(III) catalysis in conjunction with dioxazolone amidating reagents and isopropanol as an environmentally friendly hydride source. The reaction tolerates a wide range of functional groups and efficiently converts electron-deficient alkenes, styrenes, and 1,1-disubstituted alkenes, in addition to unactivated alkenes, to their corresponding linear amides. Mechanistic studies reveal a reversible rhodium hydride migratory insertion step, leading to exquisite selectivity for the anti-Markovnikov product.
中文翻译:

使用二恶唑酮作为酰胺化试剂,铑 (III) 催化的未活化烯烃的反马尔可夫尼科夫氢酰胺化反应
酰胺是所有药物中最常见的官能团之一,因此,引入酰胺部分的反应具有特别的价值。烯烃的分子间加氢酰胺化仍然是一种尚未充分探索的合成含酰胺化合物的方法。大多数氢酰胺化过程表现出马尔可夫尼科夫区域选择性,而目前的反马尔可夫尼科夫氢酰胺化方法在某种程度上仅限于活化的烯烃底物或自由基过程。在此,我们报告了一种在温和条件下对未活化的烯烃进行分子间反马可夫尼科夫氢酰胺化的通用方法,利用Rh(III)催化结合二恶唑酮酰胺化试剂和异丙醇作为环境友好的氢化物源。该反应可耐受多种官能团,可有效地将缺电子烯烃、苯乙烯和 1,1-二取代烯烃以及未活化的烯烃转化为相应的直链酰胺。机理研究揭示了可逆的氢化铑迁移插入步骤,从而导致抗马可夫尼科夫产物具有精细的选择性。
更新日期:2022-12-01
中文翻译:

使用二恶唑酮作为酰胺化试剂,铑 (III) 催化的未活化烯烃的反马尔可夫尼科夫氢酰胺化反应
酰胺是所有药物中最常见的官能团之一,因此,引入酰胺部分的反应具有特别的价值。烯烃的分子间加氢酰胺化仍然是一种尚未充分探索的合成含酰胺化合物的方法。大多数氢酰胺化过程表现出马尔可夫尼科夫区域选择性,而目前的反马尔可夫尼科夫氢酰胺化方法在某种程度上仅限于活化的烯烃底物或自由基过程。在此,我们报告了一种在温和条件下对未活化的烯烃进行分子间反马可夫尼科夫氢酰胺化的通用方法,利用Rh(III)催化结合二恶唑酮酰胺化试剂和异丙醇作为环境友好的氢化物源。该反应可耐受多种官能团,可有效地将缺电子烯烃、苯乙烯和 1,1-二取代烯烃以及未活化的烯烃转化为相应的直链酰胺。机理研究揭示了可逆的氢化铑迁移插入步骤,从而导致抗马可夫尼科夫产物具有精细的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号