当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prediction of High-Yielding Single-Step or Cascade Pericyclic Reactions for the Synthesis of Complex Synthetic Targets
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-30 , DOI: 10.1021/jacs.2c09830 Tsuyoshi Mita 1, 2 , Hideaki Takano 1, 2 , Hiroki Hayashi 1, 2 , Wataru Kanna 3 , Yu Harabuchi 1, 2, 3 , K N Houk 4 , Satoshi Maeda 1, 2, 3, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-30 , DOI: 10.1021/jacs.2c09830 Tsuyoshi Mita 1, 2 , Hideaki Takano 1, 2 , Hiroki Hayashi 1, 2 , Wataru Kanna 3 , Yu Harabuchi 1, 2, 3 , K N Houk 4 , Satoshi Maeda 1, 2, 3, 5
Affiliation
|
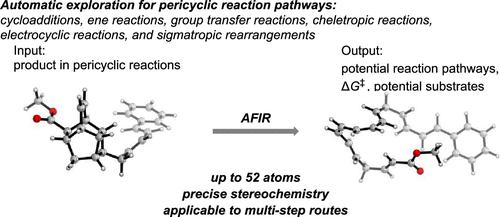
Pericyclic reactions, which involve cyclic concerted transition states without ionic or radical intermediates, have been extensively studied since their definition in the 1960s, and the famous Woodward–Hoffmann rules predict their stereoselectivity and chemoselectivity. Here, we describe the application of a fully automated reaction-path search method, that is, the artificial force induced reaction (AFIR), to trace an input compound back to reasonable starting materials through thermally allowed pericyclic reactions via product-based quantum-chemistry-aided retrosynthetic analysis (QCaRA) without using any a priori experimental knowledge. All categories of pericyclic reactions, including cycloadditions, ene reactions, group-transfer, cheletropic, electrocyclic, and sigmatropic reactions, were successfully traced back via concerted reaction pathways, and starting materials were computationally obtained with the correct stereochemistry. Furthermore, AFIR was used to predict whether the identified reaction pathway can be expected to occur in good yield relative to other possible reactions of the identified starting material. In order to showcase its practical utility, this state-of-the-art technology was also applied to the retrosynthetic analysis of a natural product with a relatively high number of atoms (52 atoms: endiandric acid C methyl ester), which was first synthesized by Nicolaou in 1982 and provided the corresponding starting polyenes with the correct stereospecificity via three pericyclic reaction cascades (one Diels–Alder reaction as well as 6π and 8π electrocyclic reactions). Moreover, not only systems that obey the Woodward–Hoffmann rules but also systems that violate these rules, such as those recently calculated by Houk, can be retrosynthesized accurately.
中文翻译:

预测用于合成复杂合成目标的高产单步或级联周环反应
周环反应涉及没有离子或自由基中间体的循环协同过渡态,自 1960 年代定义以来已被广泛研究,著名的伍德沃德-霍夫曼规则预测了它们的立体选择性和化学选择性。在这里,我们描述了一种全自动反应路径搜索方法的应用,即人工力诱导反应 (AFIR),通过基于产物的量子化学通过热允许的周环反应将输入化合物追溯到合理的起始材料-辅助逆合成分析 (QCaRA),无需使用任何先验实验知识。所有类别的周环反应,包括环加成、烯反应、基团转移、螯合反应、电环反应和 sigmatropic 反应,通过协同反应途径成功追溯,并通过计算获得具有正确立体化学的起始材料。此外,AFIR 用于预测相对于已识别的起始材料的其他可能反应,是否可以预期已识别的反应途径以良好的产率发生。为了展示其实用性,这项最先进的技术还被应用于逆合成分析一种原子数相对较高的天然产物(52个原子:二氢二氢萘酸C甲酯),该天然产物首次被合成由 Nicolaou 于 1982 年提出,并通过三个周环反应级联(一个 Diels-Alder 反应以及 6π 和 8π 电环反应)为相应的起始多烯提供了正确的立体特异性。而且,
更新日期:2022-11-30
中文翻译:

预测用于合成复杂合成目标的高产单步或级联周环反应
周环反应涉及没有离子或自由基中间体的循环协同过渡态,自 1960 年代定义以来已被广泛研究,著名的伍德沃德-霍夫曼规则预测了它们的立体选择性和化学选择性。在这里,我们描述了一种全自动反应路径搜索方法的应用,即人工力诱导反应 (AFIR),通过基于产物的量子化学通过热允许的周环反应将输入化合物追溯到合理的起始材料-辅助逆合成分析 (QCaRA),无需使用任何先验实验知识。所有类别的周环反应,包括环加成、烯反应、基团转移、螯合反应、电环反应和 sigmatropic 反应,通过协同反应途径成功追溯,并通过计算获得具有正确立体化学的起始材料。此外,AFIR 用于预测相对于已识别的起始材料的其他可能反应,是否可以预期已识别的反应途径以良好的产率发生。为了展示其实用性,这项最先进的技术还被应用于逆合成分析一种原子数相对较高的天然产物(52个原子:二氢二氢萘酸C甲酯),该天然产物首次被合成由 Nicolaou 于 1982 年提出,并通过三个周环反应级联(一个 Diels-Alder 反应以及 6π 和 8π 电环反应)为相应的起始多烯提供了正确的立体特异性。而且,











































 京公网安备 11010802027423号
京公网安备 11010802027423号