当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organic Acid to Nitrile: A Chemoenzymatic Three-Step Route
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-30 , DOI: 10.1002/adsc.202201053 Margit Winkler 1, 2 , Melissa Horvat 1 , Astrid Schiefer 3 , Victoria Weilch 1 , Florian Rudroff 3 , Miroslav Pátek 4 , Ludmila Martínková 4
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-30 , DOI: 10.1002/adsc.202201053 Margit Winkler 1, 2 , Melissa Horvat 1 , Astrid Schiefer 3 , Victoria Weilch 1 , Florian Rudroff 3 , Miroslav Pátek 4 , Ludmila Martínková 4
Affiliation
|
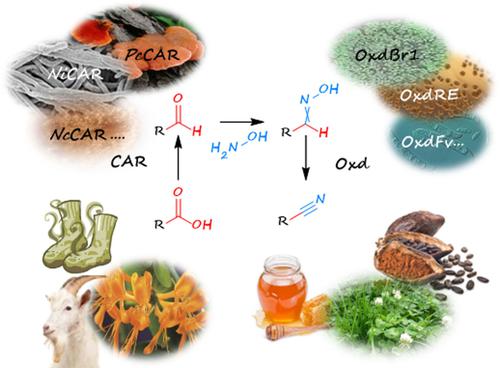
Various widely applied compounds contain cyano-groups, and this functional group serves as a chemical handle for a whole range of different reactions. We report a cyanide free chemoenzymatic cascade for nitrile synthesis. The reaction pathway starts with a reduction of carboxylic acid to aldehyde by carboxylate reductase enzymes (CARs) applied as living cell biocatalysts. The second – chemical – step includes in situ oxime formation with hydroxylamine. The final direct step from oxime to nitrile is catalyzed by aldoxime dehydratases (Oxds). With compatible combinations of a CAR and an Oxd, applied in one-pot two-step reactions, several aliphatic and aryl-aliphatic target nitriles were obtained in more than 80% conversion. Phenylacetonitrile, for example, was prepared in 78% isolated yield. This chemoenzymatic route does not require cyanide salts, toxic metals, or undesired oxidants in contrast to entirely chemical procedures.
中文翻译:

有机酸制腈:化学酶促三步路线
各种广泛应用的化合物都含有氰基,该官能团可作为各种不同反应的化学手柄。我们报告了用于腈合成的无氰化物化学酶促级联。反应途径始于通过用作活细胞生物催化剂的羧酸还原酶 (CAR) 将羧酸还原为醛。第二个——化学——步骤包括原位与羟胺形成肟。从肟到腈的最后直接步骤是由醛肟脱水酶 (Oxds) 催化的。使用 CAR 和 Oxd 的相容组合,应用于一锅两步反应,以超过 80% 的转化率获得了几种脂肪族和芳基-脂肪族目标腈。例如,苯乙腈的分离产率为 78%。与完全化学过程相比,这种化学酶促途径不需要氰化物盐、有毒金属或不需要的氧化剂。
更新日期:2022-11-30
中文翻译:

有机酸制腈:化学酶促三步路线
各种广泛应用的化合物都含有氰基,该官能团可作为各种不同反应的化学手柄。我们报告了用于腈合成的无氰化物化学酶促级联。反应途径始于通过用作活细胞生物催化剂的羧酸还原酶 (CAR) 将羧酸还原为醛。第二个——化学——步骤包括原位与羟胺形成肟。从肟到腈的最后直接步骤是由醛肟脱水酶 (Oxds) 催化的。使用 CAR 和 Oxd 的相容组合,应用于一锅两步反应,以超过 80% 的转化率获得了几种脂肪族和芳基-脂肪族目标腈。例如,苯乙腈的分离产率为 78%。与完全化学过程相比,这种化学酶促途径不需要氰化物盐、有毒金属或不需要的氧化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号