当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mn-Mediated Radical Cascade Cyclization of 1,6-Enynes with Arylboronic Acids to Access Dihydrobenzo[b]fluorenones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-30 , DOI: 10.1002/adsc.202201054 Chada Raji Reddy 1 , Roshan Chandrakant Kajare 1 , Punna Nagender 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-30 , DOI: 10.1002/adsc.202201054 Chada Raji Reddy 1 , Roshan Chandrakant Kajare 1 , Punna Nagender 2
Affiliation
|
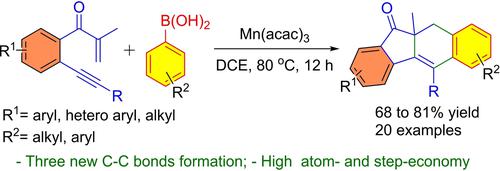
Herein, we report the synthesis of functionalized dihydro-benzo[b]fluorenones through a manganese-mediated cascade radical cyclization of β-alkynyl propenones [1,6-enynes] with arylboronic acids. In the present strategy, the in-situ generated aryl radical undergoes chemo-selective addition followed by 5-exo-trig cyclization. The reaction is emphasized by high atom- and step-economy with the construction of three new C−C bonds to access the dihydrobenzo[b]fluorenones in 68–81% yield under mild reaction conditions. The synthetic efficacy of the developed method is evidenced by the construction of new C−C and C−N bonds.
中文翻译:

锰介导的 1,6-烯炔与芳基硼酸的自由基级联环化得到二氢苯并 [b] 芴酮
在此,我们报道了通过锰介导的β-炔基丙烯酮 [1,6-烯炔] 与芳基硼酸的级联自由基环化反应,合成了功能化二氢苯并 [ b ] 芴酮。在本策略中,原位生成的芳基自由基经过化学选择性加成,然后进行 5 -exo-trig环化。该反应强调了高原子经济性和步骤经济性,通过构建三个新的 C-C 键,在温和的反应条件下以 68-81% 的产率获得二氢苯并 [ b ] 芴酮。新 C−C 和 C−N 键的构建证明了所开发方法的合成功效。
更新日期:2022-11-30
中文翻译:

锰介导的 1,6-烯炔与芳基硼酸的自由基级联环化得到二氢苯并 [b] 芴酮
在此,我们报道了通过锰介导的β-炔基丙烯酮 [1,6-烯炔] 与芳基硼酸的级联自由基环化反应,合成了功能化二氢苯并 [ b ] 芴酮。在本策略中,原位生成的芳基自由基经过化学选择性加成,然后进行 5 -exo-trig环化。该反应强调了高原子经济性和步骤经济性,通过构建三个新的 C-C 键,在温和的反应条件下以 68-81% 的产率获得二氢苯并 [ b ] 芴酮。新 C−C 和 C−N 键的构建证明了所开发方法的合成功效。









































 京公网安备 11010802027423号
京公网安备 11010802027423号