当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic oxa-Michael/Michael Addition/Deformylation Cascade Reaction of 3-Formylchromone with p-Quinol: Synthesis of the Furanochromanone Skeleton
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-30 , DOI: 10.1002/adsc.202201145 Xiurong Zheng 1 , Jiajia Chen 2 , Zhuowen Li 1 , Lin Zhong 1 , Ruoting Zhan 1 , Huicai Huang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-30 , DOI: 10.1002/adsc.202201145 Xiurong Zheng 1 , Jiajia Chen 2 , Zhuowen Li 1 , Lin Zhong 1 , Ruoting Zhan 1 , Huicai Huang 1
Affiliation
|
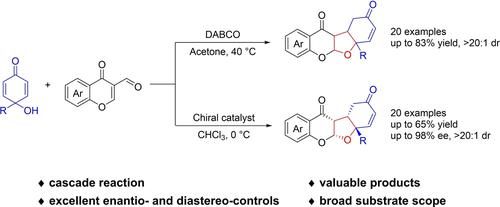
An approach for the construction of furanochromanone skeletons has been developed through a DABCO-catalyzed cascade reaction with good yields (up to 83%) and high diastereoselectivity (>20:1 dr). Notably, the asymmetric catalytic version of this reaction was further studied, obtaining furanochromanones bearing four contiguous stereogenic centers with excellent enantio- and diastereoselectivity (up to 98% ee, >20:1 dr). This protocol causes the formation of a variety of substituted furanochromanones via an oxa-Michael/Michael addition/deformylation cascade reaction.
中文翻译:

有机催化 oxa-Michael/Michael 加成/脱甲酰化级联反应 3-甲酰色酮与对羟基苯甲酸:呋喃色满酮骨架的合成
通过 DABCO 催化的级联反应开发了一种构建呋喃并色满酮骨架的方法,具有良好的产率(高达 83%)和高非对映选择性(>20:1 dr)。值得注意的是,该反应的不对称催化形式得到了进一步研究,获得了带有四个连续立体中心的呋喃并色满酮,具有出色的对映和非对映选择性(高达 98% ee,>20:1 dr)。该协议通过 oxa-Michael/Michael 添加/脱甲酰化级联反应导致形成各种取代的 furanochromanones。
更新日期:2022-11-30
中文翻译:

有机催化 oxa-Michael/Michael 加成/脱甲酰化级联反应 3-甲酰色酮与对羟基苯甲酸:呋喃色满酮骨架的合成
通过 DABCO 催化的级联反应开发了一种构建呋喃并色满酮骨架的方法,具有良好的产率(高达 83%)和高非对映选择性(>20:1 dr)。值得注意的是,该反应的不对称催化形式得到了进一步研究,获得了带有四个连续立体中心的呋喃并色满酮,具有出色的对映和非对映选择性(高达 98% ee,>20:1 dr)。该协议通过 oxa-Michael/Michael 添加/脱甲酰化级联反应导致形成各种取代的 furanochromanones。











































 京公网安备 11010802027423号
京公网安备 11010802027423号