当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
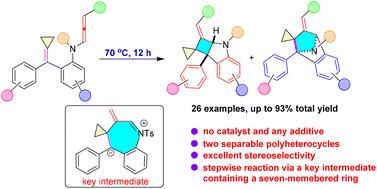
Thermally-induced intramolecular [2 + 2] cycloaddition of allene-methylenecyclopropanes: expedient access to two separable spiropolycyclic heterocycles
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-12-01 , DOI: 10.1039/d2qo01473d Min Li 1 , Yin Wei 2 , Min Shi 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-12-01 , DOI: 10.1039/d2qo01473d Min Li 1 , Yin Wei 2 , Min Shi 1, 2
Affiliation
|
In this paper, a thermally-induced intramolecular [2 + 2] cycloaddition of allene-methylenecyclopropanes has been developed for the rapid construction of nitrogen-containing spiro[bicyclo[3.2.0]heptane-6,1′-cyclopropane] and spiroheptane-2,1′-cyclopropane polycyclic heterocycles in excellent total yields with good functional group compatibility under relatively mild conditions. These two heterocycles are separable and formed at the same time in the absence of any catalyst or additive. The reaction mechanism is clarified by control experiments and DFT calculations, and it was found that the reaction proceeded through a stepwise process via a key intermediate containing a seven-membered ring rather than a concerted pathway.
中文翻译:

丙二烯-亚甲基环丙烷的热诱导分子内 [2 + 2] 环加成:方便获得两个可分离的螺多环杂环
在本文中,开发了丙二烯-亚甲基环丙烷的热诱导分子内 [2 + 2] 环加成反应,用于快速构建含氮螺[双环[3.2.0]庚烷-6,1'-环丙烷]和螺庚烷- 2,1'-环丙烷多环杂环在相对温和的条件下具有优异的总收率和良好的官能团相容性。这两个杂环在没有任何催化剂或添加剂的情况下是可分离的并同时形成。通过控制实验和DFT计算阐明了反应机理,发现反应通过包含七元环的关键中间体而不是协同途径通过逐步过程进行。
更新日期:2022-12-01
中文翻译:

丙二烯-亚甲基环丙烷的热诱导分子内 [2 + 2] 环加成:方便获得两个可分离的螺多环杂环
在本文中,开发了丙二烯-亚甲基环丙烷的热诱导分子内 [2 + 2] 环加成反应,用于快速构建含氮螺[双环[3.2.0]庚烷-6,1'-环丙烷]和螺庚烷- 2,1'-环丙烷多环杂环在相对温和的条件下具有优异的总收率和良好的官能团相容性。这两个杂环在没有任何催化剂或添加剂的情况下是可分离的并同时形成。通过控制实验和DFT计算阐明了反应机理,发现反应通过包含七元环的关键中间体而不是协同途径通过逐步过程进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号