当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Negatively Charged Iron-Bridged Fullerene Dimer {Fe(CO)2-μ2-η2,η2-C60}22–
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.inorgchem.2c03595 Maxim A Faraonov 1 , Alexey V Kuzmin 2 , Salavat S Khasanov 2 , Alexander F Shestakov 1 , Akihiro Otsuka 3 , Hideki Yamochi 3 , Hiroshi Kitagawa 3 , Dmitri V Konarev 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.inorgchem.2c03595 Maxim A Faraonov 1 , Alexey V Kuzmin 2 , Salavat S Khasanov 2 , Alexander F Shestakov 1 , Akihiro Otsuka 3 , Hideki Yamochi 3 , Hiroshi Kitagawa 3 , Dmitri V Konarev 1
Affiliation
|
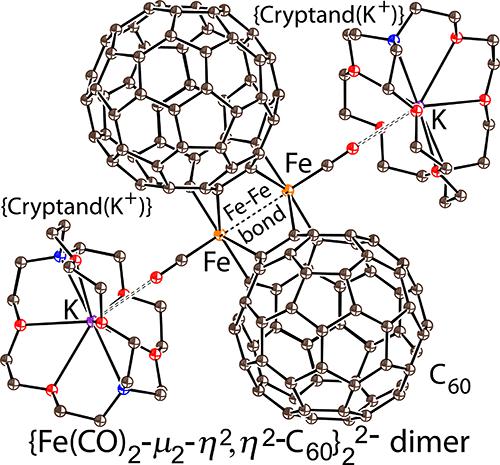
The interaction of {Cryptand(K+)}(C60•–) with Fe3(CO)12 produced {Cryptand(K+)}2{Fe(CO)2-μ2-η2,η2-C60}22–·2.5C6H4Cl2 (1) as the first negatively charged iron-bridged fullerene C60 dimer. The bridged iron atoms are coordinated to two 6–6 bonds of one C60 hexagon with short and long C(C60)–Fe bonds with average lengths of 2.042(3) and 2.088(3) Å. Fullerenes are close to each other in the dimer with a center-to-center interfullerene distance of 10.02 Å. Optical spectra support the localization of negative electron density on the Fe2(CO)4 units, which causes a 50 cm–1 shift of the C≡O vibration bands to smaller wavenumbers, and the C60 cages. Dimers are diamagnetic and electron paramagnetic resonance silent and have a singlet ground state resulting from the formation of an Fe–Fe bond in the dimer with a length of 2.978(4) Å. According to density functional theory calculations, the excited triplet state is higher than the ground state by 6.5 kcal/mol. Compound 1 shows a broad near-infrared band with a maximum at 970 nm, which is attributable to the charge transfer from the orbitals localized mainly on iron atoms to the C60 ligand.
中文翻译:

带负电的铁桥富勒烯二聚体 {Fe(CO)2-μ2-η2,η2-C60}22–
{Cryptand(K + )}(C 60 •– ) 与 Fe 3 (CO) 12的相互作用产生 {Cryptand(K + )} 2 {Fe(CO) 2 - μ 2 - η 2 , η 2 -C 60 } 2 2– ·2.5C 6 H 4 Cl 2 ( 1 ) 作为第一个带负电荷的铁桥富勒烯 C 60二聚体。桥接的铁原子与一个 C 60六边形的两个 6-6 键配位,短 C 和长 C (C 60)–Fe 键的平均长度为 2.042(3) 和 2.088(3) Å。富勒烯在二聚体中彼此靠近,富勒烯中心到中心的间距为 10.02 Å。光谱支持负电子密度在 Fe 2 (CO) 4单元上的定位,这会导致C≡O 振动带向较小的波数和 C 60笼移动50 cm –1 。二聚体是抗磁和电子顺磁共振沉默的,并且由于在二聚体中形成长度为 2.978(4) Å 的 Fe-Fe 键而具有单线态基态。根据密度泛函理论计算,激发三重态比基态高6.5 kcal/mol。化合物1显示出在 970 nm 处具有最大值的宽近红外波段,这归因于电荷从主要位于铁原子上的轨道转移到 C 60配体。
更新日期:2022-11-30
中文翻译:

带负电的铁桥富勒烯二聚体 {Fe(CO)2-μ2-η2,η2-C60}22–
{Cryptand(K + )}(C 60 •– ) 与 Fe 3 (CO) 12的相互作用产生 {Cryptand(K + )} 2 {Fe(CO) 2 - μ 2 - η 2 , η 2 -C 60 } 2 2– ·2.5C 6 H 4 Cl 2 ( 1 ) 作为第一个带负电荷的铁桥富勒烯 C 60二聚体。桥接的铁原子与一个 C 60六边形的两个 6-6 键配位,短 C 和长 C (C 60)–Fe 键的平均长度为 2.042(3) 和 2.088(3) Å。富勒烯在二聚体中彼此靠近,富勒烯中心到中心的间距为 10.02 Å。光谱支持负电子密度在 Fe 2 (CO) 4单元上的定位,这会导致C≡O 振动带向较小的波数和 C 60笼移动50 cm –1 。二聚体是抗磁和电子顺磁共振沉默的,并且由于在二聚体中形成长度为 2.978(4) Å 的 Fe-Fe 键而具有单线态基态。根据密度泛函理论计算,激发三重态比基态高6.5 kcal/mol。化合物1显示出在 970 nm 处具有最大值的宽近红外波段,这归因于电荷从主要位于铁原子上的轨道转移到 C 60配体。










































 京公网安备 11010802027423号
京公网安备 11010802027423号