European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-11-28 , DOI: 10.1016/j.ejmech.2022.114947 Wen Peng 1 , Fuyao Liu 1 , Lei Zhang 1 , Liying Zhang 2 , Jing Li 1
|
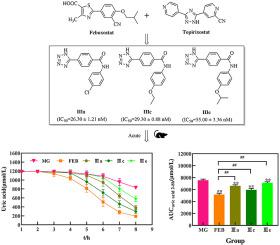
Based on analyses of the interaction between febuxostat and xanthine oxidoreductase (XOR), tetrazole was used to replace the carboxyl-thiazole fragment of febuxostat using a bioelectronic isosteric strategy. Three series of compounds were designed. The inhibitory activity against XOR of all compounds was evaluated and their structure–activity relationships determined. The inhibitory activity against XOR of compounds I was weak, with a half-maximal inhibitory concentration (IC50) value > 10 μmol, whereas the inhibitory activity of compounds II and III was increased significantly, among which compounds IIIa (IC50 = 26.3 ± 1.21 nM) and IIIc (IC50 = 29.3 ± 0.88 nM) were the best. Molecular docking showed that tetrazole could enter the active cavity instead of a carboxyl group and retain most of the interaction between febuxostat and XOR. For compounds III, the hydrogen bonds with Asn768 and Thr1010 of XOR were absent, but some new interactions were introduced to improve potency. A potassium oxazinate/hypoxanthine-induced model of acute hyperuricemia in mice also showed a significant hypouricemia effect of compounds IIIa, IIIc, and IIIe (P < 0.01), which was consistent with the results of inhibition in vitro. In conclusion, we identified a promising XOR inhibitor and provided new ideas for the design of XOR inhibitors.
中文翻译:

异或抑制剂苯基四唑三环化合物的设计、合成与评价
基于对非布索坦与黄嘌呤氧化还原酶 (XOR) 之间相互作用的分析,采用生物电子等排策略,使用四唑替代非布司他的羧基-噻唑片段。设计了三个系列的化合物。评估了所有化合物对 XOR 的抑制活性,并确定了它们的结构-活性关系。化合物I对XOR的抑制活性较弱,半数抑制浓度(IC 50)值>10 μmol,而化合物II和III的抑制活性显着增加,其中化合物IIIa(IC 50 = 26.3 ± 1.21 纳米)和IIIc(IC 50 = 29.3 ± 0.88 nM) 是最好的。分子对接表明,四唑可以进入活性腔而不是羧基,并保留了非布索坦和 XOR 之间的大部分相互作用。对于化合物III,不存在与异或的 Asn768 和 Thr1010 的氢键,但引入了一些新的相互作用以提高效力。恶嗪酸钾/次黄嘌呤诱导的小鼠急性高尿酸血症模型也显示化合物IIIa、IIIc和IIIe具有显着的低尿酸血症作用(P < 0.01),这与体外 抑制的结果一致。总之,我们确定了一种有前途的 XOR 抑制剂,并为 XOR 抑制剂的设计提供了新思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号