当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic Carbene Organocatalyzed Redox-Active/Ring Expansion Reactions: Mechanistic Insights Unveiling Base Cooperativity
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.joc.2c02462 Rozhin Rowshanpour 1 , Michel Gravel 2 , Travis Dudding 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.joc.2c02462 Rozhin Rowshanpour 1 , Michel Gravel 2 , Travis Dudding 1
Affiliation
|
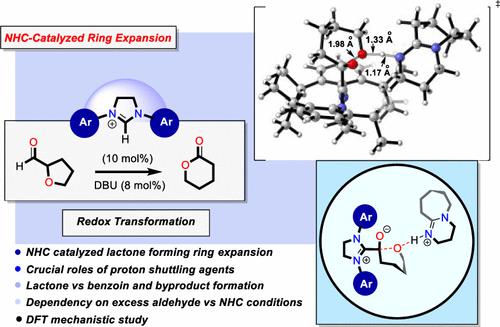
N-Heterocyclic carbene (NHC) organocatalyzed transformations of redox-active chemical manifolds is a powerful strategy for interconverting and expanding the chemical space. This approach in the context of ring expansion holds promise for preparing lactones from plentiful redox active aldehydes, despite a lack of rigorous mechanistic insights into the underlying elements governing this reactivity and with-it relevance to other NHC organocatalyzed transformations. Herein, in investigating this reactivity under the lens of modern day quantum mechanical calculations, we explore the mechanism of redox-active/ring expansion reactions of aldehydes furnishing lactone products by means of NHC organocatalysis. Through this comprehensive study, the underpinning mechanism of Breslow intermediate formation and ensuing downstream processes such as intermolecular C–C bond formation providing benzoin products versus intramolecular redox pathways are outlined. Notably, this study of NHC organocatalysis reveals the diverse role of bases as cooperative agents in directing and selectively routing reactivity, as highlighted here toward ring expanded lactone products.
中文翻译:

N-杂环卡宾有机催化的氧化还原活性/环扩展反应:揭示基础协同性的机理见解
氧化还原活性化学流形的 N-杂环卡宾 (NHC) 有机催化转化是相互转化和扩展化学空间的有力策略。尽管缺乏对控制这种反应性的基本元素及其与其他 NHC 有机催化转化的相关性的严格机制洞察,但在环扩展的背景下,这种方法有望从丰富的氧化还原活性醛中制备内酯。在此,在现代量子力学计算的镜头下研究这种反应性时,我们探索了通过 NHC 有机催化提供内酯产品的醛的氧化还原活性/扩环反应的机制。通过这次全面的学习,概述了 Breslow 中间体形成的基础机制和随后的下游过程,例如分子间 C-C 键形成提供安息香产品与分子内氧化还原途径。值得注意的是,这项关于 NHC 有机催化的研究揭示了碱基作为协同剂在指导和选择性路由反应性方面的不同作用,正如这里强调的对环扩展内酯产品。
更新日期:2022-11-30
中文翻译:

N-杂环卡宾有机催化的氧化还原活性/环扩展反应:揭示基础协同性的机理见解
氧化还原活性化学流形的 N-杂环卡宾 (NHC) 有机催化转化是相互转化和扩展化学空间的有力策略。尽管缺乏对控制这种反应性的基本元素及其与其他 NHC 有机催化转化的相关性的严格机制洞察,但在环扩展的背景下,这种方法有望从丰富的氧化还原活性醛中制备内酯。在此,在现代量子力学计算的镜头下研究这种反应性时,我们探索了通过 NHC 有机催化提供内酯产品的醛的氧化还原活性/扩环反应的机制。通过这次全面的学习,概述了 Breslow 中间体形成的基础机制和随后的下游过程,例如分子间 C-C 键形成提供安息香产品与分子内氧化还原途径。值得注意的是,这项关于 NHC 有机催化的研究揭示了碱基作为协同剂在指导和选择性路由反应性方面的不同作用,正如这里强调的对环扩展内酯产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号