当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Construction of Heteroarylation/Esterification Products of Acrylic Acids via Iridium(III)-Catalyzed C–H Activation
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03245 Jia-Xue Wu 1 , Huai-Wei Wang 1 , Wen-Zeng Duan 1 , Hong-Han Ji 1 , Jian-Min Dou 1 , Xian-Qiang Huang 1 , Yi Lu 2 , Da-Cheng Li 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-30 , DOI: 10.1021/acs.orglett.2c03245 Jia-Xue Wu 1 , Huai-Wei Wang 1 , Wen-Zeng Duan 1 , Hong-Han Ji 1 , Jian-Min Dou 1 , Xian-Qiang Huang 1 , Yi Lu 2 , Da-Cheng Li 1
Affiliation
|
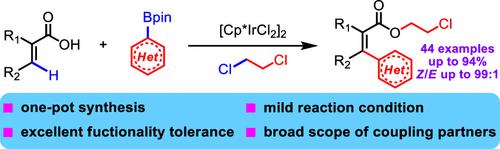
A carboxylate-assisted iridium(III)-catalyzed regioselective C(sp2)–H heteroarylation/esterification reaction of acrylic acid is disclosed herein for the first time. This catalytic protocol tolerates various α-substituted, β-substituted, and α, β-disubstituted acrylic acids as well as heteroaromatic boronates well. The resulting 3,4-dihydro-2H-pyran-6-carboxylic acid derivative 3r highlighted the AIE-active luminophore with multiple emission signal properties and a high quantum yield of 28%, exhibiting the potential application of this methodology for the synthesis of nitrogen-containing organic functional materials.
中文翻译:

通过铱 (III) 催化的 C–H 活化一锅法构建丙烯酸的杂芳基化/酯化产物
本文首次公开了羧酸盐辅助的铱 (III) 催化的区域选择性 C(sp 2 )–H 杂芳基化/酯化反应。该催化方案可以很好地耐受各种 α-取代、β-取代和 α, β-二取代丙烯酸以及杂芳族硼酸酯。由此产生的 3,4-dihydro-2 H -pyran-6-carboxylic acid 衍生物3r突出了具有多重发射信号特性和 28% 的高量子产率的 AIE 活性发光团,展示了该方法在合成含氮有机功能材料。
更新日期:2022-11-30
中文翻译:

通过铱 (III) 催化的 C–H 活化一锅法构建丙烯酸的杂芳基化/酯化产物
本文首次公开了羧酸盐辅助的铱 (III) 催化的区域选择性 C(sp 2 )–H 杂芳基化/酯化反应。该催化方案可以很好地耐受各种 α-取代、β-取代和 α, β-二取代丙烯酸以及杂芳族硼酸酯。由此产生的 3,4-dihydro-2 H -pyran-6-carboxylic acid 衍生物3r突出了具有多重发射信号特性和 28% 的高量子产率的 AIE 活性发光团,展示了该方法在合成含氮有机功能材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号