当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Functionalization of Tertiary Propargylic Boronic Esters by Alkynyllithium-Mediated 1,2-Metalate Rearrangement of Borylated Cyclopropanes
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.orglett.2c03756 Tereza Pavlíčková 1 , Yannick Stöckl 1 , Ilan Marek 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.orglett.2c03756 Tereza Pavlíčková 1 , Yannick Stöckl 1 , Ilan Marek 1
Affiliation
|
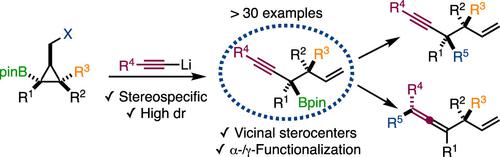
Implementing the use of alkynyllithium reagents in a stereospecific 1,2-metalate rearrangement-mediated ring opening of polysubstituted cyclopropyl boronic esters provides a variety of tertiary pinacol boranes bearing adjacent tertiary or quaternary carbon stereocenters with high levels of diastereomeric purity. The potential of this strategy was demonstrated through a selection of α- and γ-functionalization of the propargyl boronic esters.
中文翻译:

炔基锂介导的硼酸化环丙烷的 1,2-金属重排对叔炔丙基硼酸酯的合成与功能化
在多取代环丙基硼酸酯的立体特异性 1,2-金属酸酯重排介导的开环中使用炔基锂试剂提供了各种带有相邻叔或季碳立构中心的叔频哪醇硼烷,具有高水平的非对映体纯度。通过选择炔丙基硼酸酯的 α- 和 γ- 官能化,证明了该策略的潜力。
更新日期:2022-11-29
中文翻译:

炔基锂介导的硼酸化环丙烷的 1,2-金属重排对叔炔丙基硼酸酯的合成与功能化
在多取代环丙基硼酸酯的立体特异性 1,2-金属酸酯重排介导的开环中使用炔基锂试剂提供了各种带有相邻叔或季碳立构中心的叔频哪醇硼烷,具有高水平的非对映体纯度。通过选择炔丙基硼酸酯的 α- 和 γ- 官能化,证明了该策略的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号