当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manipulating the Spin State of Fe Sites via Fe–O–Si Bridge Bonds for Enhanced Polysulfide Redox Kinetics in the Li–S Battery
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.inorgchem.2c02897 Qiang Zhang 1 , Ranxiao Ao 2, 3 , Ruijie Gao 2, 3, 4 , Huaming Yang 1, 2, 3, 4
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.inorgchem.2c02897 Qiang Zhang 1 , Ranxiao Ao 2, 3 , Ruijie Gao 2, 3, 4 , Huaming Yang 1, 2, 3, 4
Affiliation
|
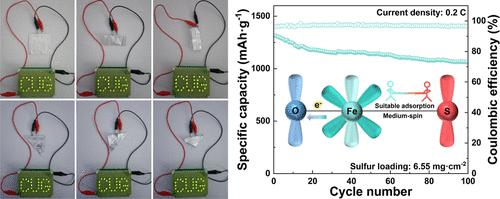
Transition metals with 3d unoccupied orbitals have superior catalytic activity, but inherent high spin suppresses their adsorption capability, leading to sluggish polysulfide conversion kinetics for Li–S batteries. Herein, we provide Fe–O–Si bridge bonds to manipulate eg filling and induce a high-to-medium spin transition of Fe3+ sites, which enhances polysulfide adsorption and facilitates sulfur redox reaction kinetics. The resultant cathodes exhibit outstanding performances under high sulfur loading, which can deliver a high battery specific energy of 1061 mA h·g–1 even after 100 cycles in Li–S pouch batteries. This work provides new insights into the kinetic and multi-step conversion mechanism of the sulfur redox reaction process, helping in the understanding and design of spin-dependent catalysts.
中文翻译:

通过 Fe-O-Si 桥键控制 Fe 位点的自旋态以增强锂硫电池中的多硫化物氧化还原动力学
具有 3d 空轨道的过渡金属具有优异的催化活性,但固有的高自旋抑制了它们的吸附能力,导致 Li-S 电池的多硫化物转化动力学缓慢。在此,我们提供 Fe-O-Si 桥键来操纵e g填充并诱导 Fe 3+位点的高到中等自旋转变,从而增强多硫化物吸附并促进硫氧化还原反应动力学。所得正极在高硫负载下表现出出色的性能,可提供 1061 mA h·g –1的高电池比能量即使在锂硫软包电池中循环 100 次之后。这项工作为硫氧化还原反应过程的动力学和多步转化机制提供了新的见解,有助于理解和设计自旋相关催化剂。
更新日期:2022-11-29
中文翻译:

通过 Fe-O-Si 桥键控制 Fe 位点的自旋态以增强锂硫电池中的多硫化物氧化还原动力学
具有 3d 空轨道的过渡金属具有优异的催化活性,但固有的高自旋抑制了它们的吸附能力,导致 Li-S 电池的多硫化物转化动力学缓慢。在此,我们提供 Fe-O-Si 桥键来操纵e g填充并诱导 Fe 3+位点的高到中等自旋转变,从而增强多硫化物吸附并促进硫氧化还原反应动力学。所得正极在高硫负载下表现出出色的性能,可提供 1061 mA h·g –1的高电池比能量即使在锂硫软包电池中循环 100 次之后。这项工作为硫氧化还原反应过程的动力学和多步转化机制提供了新的见解,有助于理解和设计自旋相关催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号