当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectrophotometric Determination of Trace Amount of Total FeII/FeIII and Live Cell Imaging of a Carboxylato Zn(II) Coordination Polymer
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.inorgchem.2c02915 Suprava Bhunia 1 , Satyajit Halder 2 , Kaushik Naskar 1 , Basudeb Dutta 1 , Dipankar Sahoo 3 , Kuladip Jana 2 , Chittaranjan Sinha 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.inorgchem.2c02915 Suprava Bhunia 1 , Satyajit Halder 2 , Kaushik Naskar 1 , Basudeb Dutta 1 , Dipankar Sahoo 3 , Kuladip Jana 2 , Chittaranjan Sinha 1
Affiliation
|
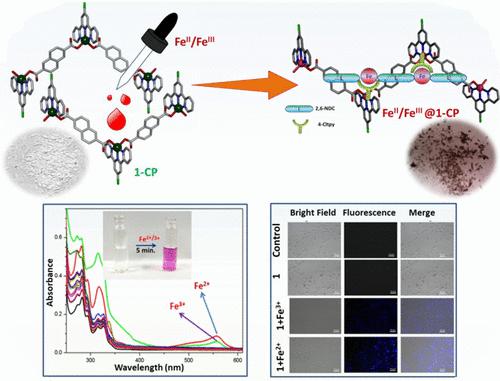
The coordination polymer, (Zn(II)-CP, 1), {[Zn(2,6-NDC)(4-Cltpy)](H2O)4} (1) (2,6-H2NDC = 2,6-naphthalene dicarboxylic acid and 4-Cltpy = 4′-chloro-[2,2′;6′,2″]terpyridine) is structurally characterized by single crystal X-ray diffraction measurement and other physicochemical studies (PXRD, FTIR, thermal analysis, microanalytical data). 4-Cltpy acts as end-capping ligand, and NDC2– is a carboxylato bridging motif to constitute ZnN3O2 distorted trigonal bipyramid core that propagates to construct 1D chain. The coordination polymer, 1, detects total iron (Fe3+ and Fe2+) in aqueous solution by visual color change, colorless to pink. Absorption spectrophotometric technique in aqueous medium measures the limit of detection (LOD) 0.11 μM (Fe2+) and 0.15 μM (Fe3+), and binding constants (Kd) are 6.7 × 104 M–1 (Fe3+) and 3.33 × 104 M–1 (Fe2+). Biocompatibility of 1 is examined in live cells, and intracellular Fe2+ and Fe3+ are detected in MDA-MB 231 cells. Zn(II) substitution is assumed upon addition of FeIII/FeII solution to the suspension of the coordination polymer, 1, in water–acetonitrile (41:1) (LZnII + FeIII/II → LFeIII + ZnII, where L is defined as coordinated ligands), which is accompanied by changing from colorless to pink at room temperature. The color of the mixture may be assumed to the charge transfer transition from carboxylate-O to Cltpy via Fe(II/III) bridging center (carboxylate–O–Fe-CltPy). The product isolated from the reaction is finally characterized as Fe(III)@1-CP. It is presumed that product Fe(II)@1-CP may undergo fast aerial oxidation to transform Fe(III)@1-CP. The FeIII exchanged framework (Fe(III)@1-CP) has been characterized by PXRD, IR, TGA and energy dispersive X-ray analysis (EDX)-SEM. The MTT assay calculates the cell viability (%), and the tolerance limit is 100 μM to total Fe2+ and Fe3+.
中文翻译:

分光光度法测定痕量总 FeII/FeIII 和羧酸锌 (II) 配位聚合物的活细胞成像
配位聚合物, (Zn(II)-CP, 1 ), {[Zn(2,6-NDC)(4-Cltpy)](H 2 O) 4 } ( 1 ) (2,6-H 2 NDC = 2,6-萘二甲酸和 4-Cltpy = 4'-氯-[2,2';6',2″]三联吡啶)通过单晶 X 射线衍射测量和其他物理化学研究(PXRD、FTIR)进行结构表征、热分析、微量分析数据)。4-Cltpy作为封端配体,NDC 2–是羧基桥接基序,构成ZnN 3 O 2畸变三角双锥核心,传播构建一维链。配位聚合物1检测总铁(Fe 3+和 Fe 2+) 在水溶液中目视变色,无色至粉红色。水性介质中的吸收分光光度法测量的检测限 (LOD) 为 0.11 μM (Fe 2+ ) 和 0.15 μM (Fe 3+ ),结合常数 (K d ) 为 6.7 × 10 4 M –1 (Fe 3+ )和 3.33 × 10 4 M –1 (Fe 2+ )。在活细胞中检查1的生物相容性,并在 MDA-MB 231 细胞中检测细胞内 Fe 2+和 Fe 3+ 。添加 Fe III /Fe II 后假定 Zn(II) 取代配位聚合物1在水-乙腈 (41:1) 中的悬浮液溶液(LZn II + Fe III/II → LFe III + Zn II,其中 L 定义为配位配体),伴随着从室温下无色至粉红色。混合物的颜色可以假设为通过 Fe(II/III) 桥接中心(羧酸盐-O-Fe-CltPy)从羧酸盐-O 到 Cltpy 的电荷转移转变。从反应中分离出的产物最终被表征为Fe(III)@1 -CP。据推测,产物Fe(II)@1 -CP 可能会经历快速空气氧化,转化为Fe(III)@1 -CP。三铁交换框架 ( Fe(III)@1 -CP) 已通过 PXRD、IR、TGA 和能量色散 X 射线分析 (EDX)-SEM 进行表征。MTT测定法计算细胞活力(%),并且对总Fe 2+和Fe 3+的耐受限度为100μM 。
更新日期:2022-11-29
中文翻译:

分光光度法测定痕量总 FeII/FeIII 和羧酸锌 (II) 配位聚合物的活细胞成像
配位聚合物, (Zn(II)-CP, 1 ), {[Zn(2,6-NDC)(4-Cltpy)](H 2 O) 4 } ( 1 ) (2,6-H 2 NDC = 2,6-萘二甲酸和 4-Cltpy = 4'-氯-[2,2';6',2″]三联吡啶)通过单晶 X 射线衍射测量和其他物理化学研究(PXRD、FTIR)进行结构表征、热分析、微量分析数据)。4-Cltpy作为封端配体,NDC 2–是羧基桥接基序,构成ZnN 3 O 2畸变三角双锥核心,传播构建一维链。配位聚合物1检测总铁(Fe 3+和 Fe 2+) 在水溶液中目视变色,无色至粉红色。水性介质中的吸收分光光度法测量的检测限 (LOD) 为 0.11 μM (Fe 2+ ) 和 0.15 μM (Fe 3+ ),结合常数 (K d ) 为 6.7 × 10 4 M –1 (Fe 3+ )和 3.33 × 10 4 M –1 (Fe 2+ )。在活细胞中检查1的生物相容性,并在 MDA-MB 231 细胞中检测细胞内 Fe 2+和 Fe 3+ 。添加 Fe III /Fe II 后假定 Zn(II) 取代配位聚合物1在水-乙腈 (41:1) 中的悬浮液溶液(LZn II + Fe III/II → LFe III + Zn II,其中 L 定义为配位配体),伴随着从室温下无色至粉红色。混合物的颜色可以假设为通过 Fe(II/III) 桥接中心(羧酸盐-O-Fe-CltPy)从羧酸盐-O 到 Cltpy 的电荷转移转变。从反应中分离出的产物最终被表征为Fe(III)@1 -CP。据推测,产物Fe(II)@1 -CP 可能会经历快速空气氧化,转化为Fe(III)@1 -CP。三铁交换框架 ( Fe(III)@1 -CP) 已通过 PXRD、IR、TGA 和能量色散 X 射线分析 (EDX)-SEM 进行表征。MTT测定法计算细胞活力(%),并且对总Fe 2+和Fe 3+的耐受限度为100μM 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号