当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Substrate-Dependent Denitrogenative Rearrangements of Rhodium Azavinyl Carbenes Involving 1,2-Aryl Migration
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.orglett.2c03538 Geetanjali S Sontakke 1 , Kuntal Pal 1 , Chandra M R Volla 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-11-29 , DOI: 10.1021/acs.orglett.2c03538 Geetanjali S Sontakke 1 , Kuntal Pal 1 , Chandra M R Volla 1
Affiliation
|
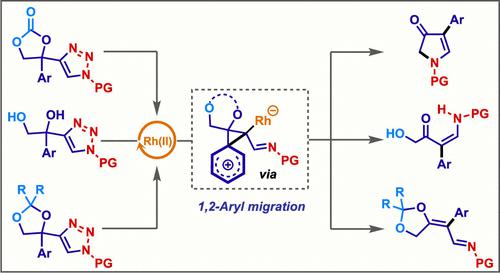
Herein, we disclose substrate-dependent rearrangements of 4-substituted N-sulfonyl-1,2,3-triazoles under Rh(II)-catalysis via denitrogenation. The reaction pathways included key 1,2-aryl migration via the formation of intermediatory phenonium ion, which is elusive so far with Rh-azavinyl carbenes. Intriguingly, the transformations were completely dependent on the substituent present leading to different scaffolds like enaminones, pyrrol-3-ones, and azadienes. Hammett studies provided essential insights into the carbocationic intermediate formation. The developed methodology featured simple reaction conditions, excellent functional group compatibility, and broad substrate scope.
中文翻译:

涉及 1,2-芳基迁移的铑氮杂乙烯基卡宾的底物依赖性脱氮重排
在此,我们公开了在 Rh(II) 催化下通过脱氮作用对 4-取代的N -磺酰基-1,2,3-三唑进行的底物依赖性重排。反应途径包括通过形成中间苯离子进行关键的 1,2-芳基迁移,到目前为止,Rh-氮杂乙烯基卡宾还难以实现这一点。有趣的是,这些转化完全取决于存在的取代基,从而导致不同的骨架,如烯胺酮、吡咯-3-酮和氮杂二烯。Hammett 研究提供了对碳阳离子中间体形成的重要见解。所开发的方法具有简单的反应条件、优异的官能团相容性和广泛的底物范围。
更新日期:2022-11-29
中文翻译:

涉及 1,2-芳基迁移的铑氮杂乙烯基卡宾的底物依赖性脱氮重排
在此,我们公开了在 Rh(II) 催化下通过脱氮作用对 4-取代的N -磺酰基-1,2,3-三唑进行的底物依赖性重排。反应途径包括通过形成中间苯离子进行关键的 1,2-芳基迁移,到目前为止,Rh-氮杂乙烯基卡宾还难以实现这一点。有趣的是,这些转化完全取决于存在的取代基,从而导致不同的骨架,如烯胺酮、吡咯-3-酮和氮杂二烯。Hammett 研究提供了对碳阳离子中间体形成的重要见解。所开发的方法具有简单的反应条件、优异的官能团相容性和广泛的底物范围。



























 京公网安备 11010802027423号
京公网安备 11010802027423号