当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
[3 + 2]-Cycloadditions with Porphyrin β,β′-Bonds: Theoretical Basis of the Counterintuitive meso-Aryl Group Influence on the Rates of Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.joc.2c02192 Remy F Lalisse 1 , Christopher M Hadad 1 , Christian Brückner 2 , Matthew J Guberman-Pfeffer 3, 4
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.joc.2c02192 Remy F Lalisse 1 , Christopher M Hadad 1 , Christian Brückner 2 , Matthew J Guberman-Pfeffer 3, 4
Affiliation
|
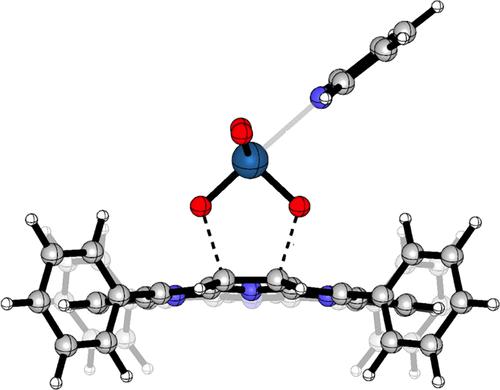
Removal of a β,β′-bond from meso-tetraarylporphyrin using [3 + 2]-cycloadditions generates meso-tetraarylhydroporphyrins. Literature evidence indicates that meso-tetraphenylporphyrins react more sluggishly with 1,3-dipoles such as ylides and OsO4 (in the presence of pyridine) than meso-tetrakis(pentafluorophenyl)porphyrin. The trend is counterintuitive for the reaction with OsO4, as this formal oxidation reaction is expected to proceed more readily with more electron-rich substrates. This work presents a density functional theory–based computational study of the frontier molecular orbital (FMO) interactions and reaction profile thermodynamics involved in the reaction of archetypical cycloaddition reactions (a simple ylide, OsO4, OsO4·py, OsO4·(py)2, and ozone) with the β,β′-double bonds of variously fluorinated meso-arylporphyrins. The trend observed for the Type I cycloaddition of an ylide is straightforward, as lowering the LUMO of the porphyrin with increasing meso-phenyl-fluorination also lowers the reaction barrier. The corresponding simple FMO analyses of Type III cycloadditions do not correctly model the reaction energetics. This is because increasing fluorination leads to lowering of the porphyrin HOMO–2, thus increasing the reaction barrier. However, coordination of pyridine to OsO4 preorganizes the transition state complex; lowering of the energy barrier by the preorganization exceeds the increase in repulsive orbital interactions, overall accelerating the cycloaddition and rationalizing the counterintuitive experimental findings.
中文翻译:

[3 + 2]-卟啉 β,β'-键的环加成:反直觉的内消旋芳基对反应速率影响的理论基础
使用 [3 + 2]-环加成从内消旋 - 四芳基卟啉中去除 β,β'- 键生成内消旋-四芳基氢卟啉。文献证据表明,内消旋- 四苯基卟啉与 1,3-偶极子如叶立德和 OsO 4(在吡啶存在下)的反应比内消旋-四(五氟苯基)卟啉更缓慢。该趋势对于与 OsO 4的反应是违反直觉的,因为这种正式的氧化反应预计会在更多富含电子的底物上更容易地进行。这项工作提出了基于密度泛函理论的前沿分子轨道 (FMO) 相互作用和反应曲线热力学的计算研究,这些研究涉及原型环加成反应(简单叶立德、OsO 4、OsO 4 ·py、OsO 4 ·(py ) 2和臭氧) 与各种氟化的内消旋-芳基卟啉的 β,β'-双键。叶立德的 I 型环加成观察到的趋势很简单,随着内观增加,卟啉的 LUMO降低-苯基氟化也降低了反应势垒。III 型环加成的相应简单 FMO 分析不能正确模拟反应能量学。这是因为增加氟化会导致卟啉 HOMO-2 降低,从而增加反应势垒。然而,吡啶与 OsO 4的配位预组织了过渡态复合物;预组织对能垒的降低超过了排斥轨道相互作用的增加,总体上加速了环加成并使违反直觉的实验结果合理化。
更新日期:2022-11-28
中文翻译:

[3 + 2]-卟啉 β,β'-键的环加成:反直觉的内消旋芳基对反应速率影响的理论基础
使用 [3 + 2]-环加成从内消旋 - 四芳基卟啉中去除 β,β'- 键生成内消旋-四芳基氢卟啉。文献证据表明,内消旋- 四苯基卟啉与 1,3-偶极子如叶立德和 OsO 4(在吡啶存在下)的反应比内消旋-四(五氟苯基)卟啉更缓慢。该趋势对于与 OsO 4的反应是违反直觉的,因为这种正式的氧化反应预计会在更多富含电子的底物上更容易地进行。这项工作提出了基于密度泛函理论的前沿分子轨道 (FMO) 相互作用和反应曲线热力学的计算研究,这些研究涉及原型环加成反应(简单叶立德、OsO 4、OsO 4 ·py、OsO 4 ·(py ) 2和臭氧) 与各种氟化的内消旋-芳基卟啉的 β,β'-双键。叶立德的 I 型环加成观察到的趋势很简单,随着内观增加,卟啉的 LUMO降低-苯基氟化也降低了反应势垒。III 型环加成的相应简单 FMO 分析不能正确模拟反应能量学。这是因为增加氟化会导致卟啉 HOMO-2 降低,从而增加反应势垒。然而,吡啶与 OsO 4的配位预组织了过渡态复合物;预组织对能垒的降低超过了排斥轨道相互作用的增加,总体上加速了环加成并使违反直觉的实验结果合理化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号