当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Thioether-Bridged 2,6-Disubstituted and 2,5,6-Trisubstituted Imidazothiadiazole Analogues: Synthesis, Antiproliferative Activity, ADME, and Molecular Docking Studies
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2022-11-29 , DOI: 10.1002/cbdv.202200884 Ibrahim Ozcan 1 , Senem Akkoc 2, 3 , Hakan Alici 4 , Seval Capanlar 5 , Onur Sahin 6 , Hakan Tahtaci 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2022-11-29 , DOI: 10.1002/cbdv.202200884 Ibrahim Ozcan 1 , Senem Akkoc 2, 3 , Hakan Alici 4 , Seval Capanlar 5 , Onur Sahin 6 , Hakan Tahtaci 1
Affiliation
|
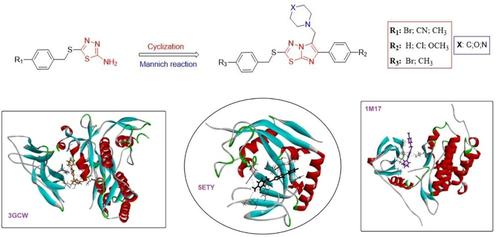
In this study, starting from 2-amino-1,3,4-thiadiazole derivatives (3–5), a new series of 2,6-disubstituted (compounds 7–15) and 2,5,6-trisubstituted (compounds 16–33) imidazo[2,1-b][1,3,4]-thiadiazole derivatives were synthesized using cyclization and Mannich reaction mechanisms, respectively. All synthesized compounds were characterized by 1H-NMR, 13C-NMR, FT-IR, elemental analysis, and mass spectroscopy techniques. Also, X-ray diffraction analysis were used for compounds 4, 7, 11, 17, and 19. The cytotoxic effects of the new compounds on the viability of colon cancer cells (DLD-1), lung cancer cells (A549), and liver cancer cells (HepG2) were investigated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method in vitro. Compound 15 was found to be the most potent anticancer drug candidate in this series with an IC50 value of 3.63 μM against HepG2 for 48 h. Moreover, the absorption, distribution, metabolism, and excretion (ADME) parameters of the synthesized compounds were calculated and thus, their potential to be safe drugs was evaluated. Finally, to support the biological activity experiments, molecular docking studies of these compounds were carried out on three different target cancer protein structures (PDB IDs: 5ETY, 1M17, and 3GCW), and the amino acids that play key roles in the binding of the compounds to these proteins were determined.
中文翻译:

新型硫醚桥接 2,6-二取代和 2,5,6-三取代咪唑并噻二唑类似物:合成、抗增殖活性、ADME 和分子对接研究
在这项研究中,从 2-amino-1,3,4-thiadiazole 衍生物 ( 3 – 5 ) 开始,一系列新的 2,6-二取代 (化合物7 – 15 ) 和 2,5,6-三取代 (化合物16 ) – 33 ) 分别使用环化和曼尼希反应机制合成了咪唑并[2,1 -b ][1,3,4]-噻二唑衍生物。所有合成的化合物都通过1 H-NMR、13 C-NMR、FT-IR、元素分析和质谱技术进行了表征。此外,X射线衍射分析用于化合物4、7、11、17和19 . 使用 3-(4,5-二甲基噻唑-2-基) 研究了新化合物对结肠癌细胞 (DLD-1)、肺癌细胞 (A549) 和肝癌细胞 (HepG2) 的细胞毒性作用)-2,5-二苯基溴化四唑 (MTT)体外法。化合物15被发现是该系列中最有效的抗癌候选药物,IC为 5048 小时内针对 HepG2 的值为 3.63 μM。此外,还计算了合成化合物的吸收、分布、代谢和排泄 (ADME) 参数,从而评估了它们作为安全药物的潜力。最后,为了支持生物活性实验,这些化合物的分子对接研究在三种不同的靶癌蛋白结构(PDB ID:5ETY、1M17 和 3GCW)以及在结合中起关键作用的氨基酸上进行。确定了这些蛋白质的化合物。
更新日期:2022-11-29
中文翻译:

新型硫醚桥接 2,6-二取代和 2,5,6-三取代咪唑并噻二唑类似物:合成、抗增殖活性、ADME 和分子对接研究
在这项研究中,从 2-amino-1,3,4-thiadiazole 衍生物 ( 3 – 5 ) 开始,一系列新的 2,6-二取代 (化合物7 – 15 ) 和 2,5,6-三取代 (化合物16 ) – 33 ) 分别使用环化和曼尼希反应机制合成了咪唑并[2,1 -b ][1,3,4]-噻二唑衍生物。所有合成的化合物都通过1 H-NMR、13 C-NMR、FT-IR、元素分析和质谱技术进行了表征。此外,X射线衍射分析用于化合物4、7、11、17和19 . 使用 3-(4,5-二甲基噻唑-2-基) 研究了新化合物对结肠癌细胞 (DLD-1)、肺癌细胞 (A549) 和肝癌细胞 (HepG2) 的细胞毒性作用)-2,5-二苯基溴化四唑 (MTT)体外法。化合物15被发现是该系列中最有效的抗癌候选药物,IC为 5048 小时内针对 HepG2 的值为 3.63 μM。此外,还计算了合成化合物的吸收、分布、代谢和排泄 (ADME) 参数,从而评估了它们作为安全药物的潜力。最后,为了支持生物活性实验,这些化合物的分子对接研究在三种不同的靶癌蛋白结构(PDB ID:5ETY、1M17 和 3GCW)以及在结合中起关键作用的氨基酸上进行。确定了这些蛋白质的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号