当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Bonding-directed Sequential 1,6/1,4-Addition of Heteroatom Nucleophiles onto Electron-deficient 1,3-Diynes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-29 , DOI: 10.1039/d2qo01730j Erandi Liyanage Perera 1 , Daesung Lee 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-29 , DOI: 10.1039/d2qo01730j Erandi Liyanage Perera 1 , Daesung Lee 1
Affiliation
|
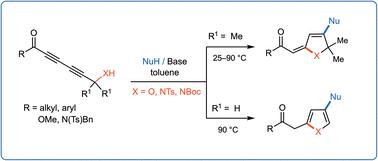
A sequential inter- and intramolecular 1,6/1,4-addition on electron-deficient 1,3-diynes conjugated with a ketone, ester, or imide functionality is described. This tandem process is effectively directed by a propargylic –OH or –NH functionality. Alcohols, amines, and thiols show excellent reactivity for the initial 1,6-addition. The addition of amines occurs at ambient temperature, whereas other nucleophiles require high temperatures up to 100 °C and a base additive such as DABCO. Substrates with propargylic hydrogen undergo double bond isomerization at higher temperatures (70–100 °C) to form furan and pyrrole rings. Nonenolizable 1,3-diynones with an electron-withdrawing group provide good yields (72–86%) with most nucleophiles.
中文翻译:

氢键定向顺序 1,6/1,4-杂原子亲核试剂加成到缺电子 1,3-二炔上
描述了与酮、酯或酰亚胺官能团结合的缺电子 1,3-二炔的顺序分子间和分子内 1,6/1,4-加成。该串联过程有效地由炔丙基 –OH 或 –NH 官能团引导。醇类、胺类和硫醇对最初的 1,6-加成表现出优异的反应性。胺的加成在环境温度下发生,而其他亲核试剂需要高达 100 °C 的高温和碱性添加剂,例如 DABCO。具有炔丙基氢的底物在较高温度 (70–100 °C) 下会发生双键异构化,形成呋喃和吡咯环。具有吸电子基团的不可烯醇化的 1,3-二炔酮对大多数亲核试剂具有良好的产率 (72–86%)。
更新日期:2022-11-30
中文翻译:

氢键定向顺序 1,6/1,4-杂原子亲核试剂加成到缺电子 1,3-二炔上
描述了与酮、酯或酰亚胺官能团结合的缺电子 1,3-二炔的顺序分子间和分子内 1,6/1,4-加成。该串联过程有效地由炔丙基 –OH 或 –NH 官能团引导。醇类、胺类和硫醇对最初的 1,6-加成表现出优异的反应性。胺的加成在环境温度下发生,而其他亲核试剂需要高达 100 °C 的高温和碱性添加剂,例如 DABCO。具有炔丙基氢的底物在较高温度 (70–100 °C) 下会发生双键异构化,形成呋喃和吡咯环。具有吸电子基团的不可烯醇化的 1,3-二炔酮对大多数亲核试剂具有良好的产率 (72–86%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号