Cell Reports ( IF 7.5 ) Pub Date : 2022-11-29 , DOI: 10.1016/j.celrep.2022.111722 Bingke Ma 1 , Xingyue Shan 1 , Juehua Yu 2 , Tailin Zhu 3 , Ren Li 4 , Hui Lv 3 , Haidi Cheng 1 , Tiantian Zhang 1 , Lihua Wang 3 , Feiyang Wei 3 , Bo Meng 5 , Xiaobing Yuan 5 , Bing Mei 5 , Xiao-Yong Zhang 4 , Wei-Guang Li 6 , Fei Li 3
|
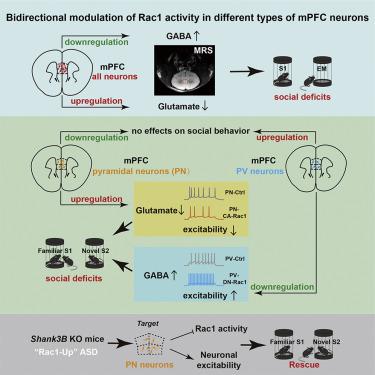
Identifying symptom-specific convergent mechanisms for neurodevelopmental disorders is a promising strategy in advancing therapies. Here, we show that bidirectional dysregulation of Rac1 activity in the medial prefrontal cortex (mPFC) dictates shared social deficits in mice. Selective upregulation or downregulation of Rac1 activity in glutamatergic or fast-spiking GABAergic neurons results in excessive or inadequate control of excitability combined with a decrease in glutamate or an increase in GABA concentrations and an increase in the GABA/glutamate ratio, which is responsible for social deficits. Notably, the autism model of Shank3B knockout mice exhibits aberrantly enhanced Rac1 activity, reduced glutamate concentrations, and pyramidal neuron excitability in mPFC accompanied with social deficits, which were corrected by either excitatory-neuron-specific downregulation of Rac1 activity or upregulation of neuronal excitability. Thus, this work shows a convergence between genetic autism risk factors, dysregulation of Rac1 signaling, and excitation-inhibition imbalance, enabling mechanism-based stratification of patients with social deficits.
中文翻译:

通过失调的前额皮质神经元的 Rac1 依赖性兴奋性控制和增加的 GABA/谷氨酸比率导致社会缺陷
确定神经发育障碍的症状特异性收敛机制是推进治疗的一个有前途的策略。在这里,我们表明内侧前额叶皮层 (mPFC) 中 Rac1 活动的双向失调决定了小鼠的共同社交缺陷。选择性上调或下调谷氨酸能或快速增加的 GABA 能神经元中的 Rac1 活性会导致兴奋性控制过度或不足,同时谷氨酸减少或 GABA 浓度增加以及 GABA/谷氨酸比率增加,这对社会活动负责赤字。值得注意的是, Shank3B的自闭症模型敲除小鼠表现出异常增强的 Rac1 活性、降低的谷氨酸浓度和 mPFC 中的锥体神经元兴奋性,并伴有社交缺陷,这些缺陷通过 Rac1 活性的兴奋性神经元特异性下调或神经元兴奋性的上调得到纠正。因此,这项工作显示了遗传自闭症风险因素、Rac1 信号失调和兴奋抑制失衡之间的趋同,从而能够对具有社会缺陷的患者进行基于机制的分层。











































 京公网安备 11010802027423号
京公网安备 11010802027423号