当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Serine Coordination in the Structural and Functional Protection of the Nitrogenase P-Cluster
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-29 , DOI: 10.1021/jacs.2c09480 Hannah L Rutledge 1 , Mackenzie J Field 2 , Jonathan Rittle 1 , Michael T Green 2, 3 , F Akif Tezcan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-29 , DOI: 10.1021/jacs.2c09480 Hannah L Rutledge 1 , Mackenzie J Field 2 , Jonathan Rittle 1 , Michael T Green 2, 3 , F Akif Tezcan 1
Affiliation
|
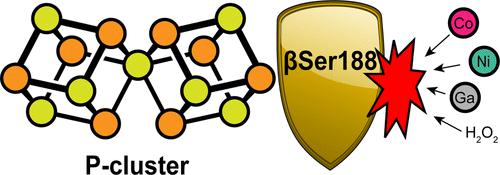
Nitrogenase catalyzes the multielectron reduction of dinitrogen to ammonia. Electron transfer in the catalytic protein (MoFeP) proceeds through a unique [8Fe-7S] cluster (P-cluster) to the active site (FeMoco). In the reduced, all-ferrous (PN) state, the P-cluster is coordinated by six cysteine residues. Upon two-electron oxidation to the P2+ state, the P-cluster undergoes conformational changes in which a highly conserved oxygen-based residue (a Ser or a Tyr) and a backbone amide additionally ligate the cluster. Previous studies of Azotobacter vinelandii (Av) MoFeP revealed that when the oxygen-based residue, βSer188, was mutated to a noncoordinating residue, Ala, the P-cluster became redox-labile and reversibly lost two of its eight Fe centers. Surprisingly, the Av strain with a MoFeP variant that lacked the serine ligand (Av βSer188Ala MoFeP) displayed the same diazotrophic growth and in vitro enzyme turnover rates as wild-type Av MoFeP, calling into question the necessity of this conserved ligand for nitrogenase function. Based on these observations, we hypothesized that βSer188 plays a role in protecting the P-cluster under nonideal conditions. Here, we investigated the protective role of βSer188 both in vivo and in vitro by characterizing the ability of Av βSer188Ala cells to grow under suboptimal conditions (high oxidative stress or Fe limitation) and by determining the tendency of βSer188Ala MoFeP to be mismetallated in vitro. Our results demonstrate that βSer188 (1) increases Av cell survival upon exposure to oxidative stress in the form of hydrogen peroxide, (2) is necessary for efficient Av diazotrophic growth under Fe-limiting conditions, and (3) may protect the P-cluster from metal exchange in vitro. Taken together, our findings suggest a structural adaptation of nitrogenase to protect the P-cluster via Ser ligation, which is a previously unidentified functional role of the Ser residue in redox proteins and adds to the expanding functional roles of non-Cys ligands to FeS clusters.
中文翻译:

丝氨酸协调在固氮酶 P 簇结构和功能保护中的作用
固氮酶催化二氮到氨的多电子还原。催化蛋白 (MoFeP) 中的电子转移通过独特的 [8Fe-7S] 簇(P 簇)到达活性位点 (FeMoco)。在还原的全铁 (P N ) 状态下,P 簇由六个半胱氨酸残基协调。在双电子氧化至 P 2+态时,P 簇会发生构象变化,其中高度保守的氧基残基(Ser 或 Tyr)和主链酰胺另外连接该簇。先前对Azotobacter vinelandii ( Av ) MoFeP 的研究表明,当基于氧的残基 βSer188 突变为非配位残基 Ala 时,P 簇变得氧化还原不稳定,并可逆地丢失其八个 Fe 中心中的两个。令人惊讶的是,具有缺乏丝氨酸配体( Av βSer188Ala MoFeP)的 MoFeP 变体的Av菌株表现出与野生型Av MoFeP 相同的固氮生长和体外酶周转率,这使人质疑这种保守配体对于固氮酶功能的必要性。基于这些观察,我们假设 βSer188 在非理想条件下发挥保护 P 簇的作用。在这里,我们通过表征Av βSer188Ala 细胞在次优条件(高氧化应激或 Fe 限制)下生长的能力以及确定 βSer188Ala MoFeP在体外错金属化的趋势,研究了 βSer188在体内和体外的保护作用。 我们的结果表明,βSer188 (1) 在暴露于过氧化氢形式的氧化应激时增加Av细胞的存活率,(2) 对于 Fe 限制条件下有效的Av固氮生长是必要的,(3) 可以保护 P 簇来自体外金属交换。综上所述,我们的研究结果表明固氮酶的结构适应通过 Ser 连接来保护 P 簇,这是氧化还原蛋白中 Ser 残基先前未识别的功能作用,并增加了非 Cys 配体对 FeS 簇的扩展功能作用。
更新日期:2022-11-29
中文翻译:

丝氨酸协调在固氮酶 P 簇结构和功能保护中的作用
固氮酶催化二氮到氨的多电子还原。催化蛋白 (MoFeP) 中的电子转移通过独特的 [8Fe-7S] 簇(P 簇)到达活性位点 (FeMoco)。在还原的全铁 (P N ) 状态下,P 簇由六个半胱氨酸残基协调。在双电子氧化至 P 2+态时,P 簇会发生构象变化,其中高度保守的氧基残基(Ser 或 Tyr)和主链酰胺另外连接该簇。先前对Azotobacter vinelandii ( Av ) MoFeP 的研究表明,当基于氧的残基 βSer188 突变为非配位残基 Ala 时,P 簇变得氧化还原不稳定,并可逆地丢失其八个 Fe 中心中的两个。令人惊讶的是,具有缺乏丝氨酸配体( Av βSer188Ala MoFeP)的 MoFeP 变体的Av菌株表现出与野生型Av MoFeP 相同的固氮生长和体外酶周转率,这使人质疑这种保守配体对于固氮酶功能的必要性。基于这些观察,我们假设 βSer188 在非理想条件下发挥保护 P 簇的作用。在这里,我们通过表征Av βSer188Ala 细胞在次优条件(高氧化应激或 Fe 限制)下生长的能力以及确定 βSer188Ala MoFeP在体外错金属化的趋势,研究了 βSer188在体内和体外的保护作用。 我们的结果表明,βSer188 (1) 在暴露于过氧化氢形式的氧化应激时增加Av细胞的存活率,(2) 对于 Fe 限制条件下有效的Av固氮生长是必要的,(3) 可以保护 P 簇来自体外金属交换。综上所述,我们的研究结果表明固氮酶的结构适应通过 Ser 连接来保护 P 簇,这是氧化还原蛋白中 Ser 残基先前未识别的功能作用,并增加了非 Cys 配体对 FeS 簇的扩展功能作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号