当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron Isotopes in Acid Mine Drainage: Extreme and Divergent Fractionation between Solid (Schwertmannite, Jarosite, and Ferric Arsenate) and Aqueous Species
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.est.2c05999 Edward D Burton 1 , Niloofar Karimian 2, 3 , Jessica L Hamilton 4 , Andrew J Frierdich 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.est.2c05999 Edward D Burton 1 , Niloofar Karimian 2, 3 , Jessica L Hamilton 4 , Andrew J Frierdich 2
Affiliation
|
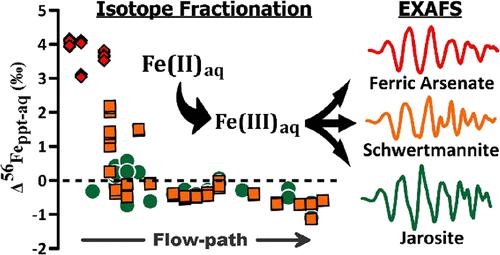
Examination of stable Fe isotopes is a powerful tool to explore Fe cycling in a range of environments. However, the isotopic fractionation of Fe in acid mine drainage (AMD) has received little attention and is poorly understood. Here, we analyze Fe isotopes in waters and Fe(III)-rich solids along an AMD flow-path. Aqueous Fe spanned a concentration and δ56Fe range of ∼420 mg L–1 and + 0.04‰ at the AMD source to ∼100 mg L–1 and −0.81‰ at ∼450 m downstream. Aqueous As (up to ∼33 mg L–1) and SO42– (up to ∼2000 mg L–1), like aqueous Fe, decreased in concentration down the flow-path. X-ray absorption spectroscopy indicated that downstream attenuation in aqueous Fe, As, and SO42– was due to the precipitation of amorphous ferric arsenate (AFA), schwertmannite, and jarosite. The Fe(III) in these solids displayed extreme variability in δ56Fe, spanning +3.95‰ in AFA near the AMD source to −1.34‰ in schwertmannite at ∼450 m downstream. Similarly, the isotopic contrast between solid Fe(III) precipitates and aqueous Fe (Δ56Feppt-aq) dropped along the flow-path from about +4.1 to −1.1‰. The shift from positive to negative Δ56Feppt-aq reflects divergence between competing equilibrium versus kinetic fractionation processes.
中文翻译:

酸性矿山排水中的铁同位素:固体(Schwertmannite、Jarosite 和砷酸铁)和水相之间的极端和发散分馏
检查稳定的 Fe 同位素是探索各种环境中 Fe 循环的有力工具。然而,酸性矿山废水 (AMD) 中 Fe 的同位素分馏很少受到关注,也知之甚少。在这里,我们分析了沿 AMD 流路的水域和富含 Fe(III) 的固体中的 Fe 同位素。含水 Fe 的浓度和 δ 56 Fe 范围从 AMD 源处的 ∼420 mg L –1和 + 0.04‰ 到下游 ∼450 m 处的∼100 mg L –1 和 −0.81‰ 。As 水溶液(高达 ∼33 mg L –1)和 SO 4 2–(高达 ∼2000 mg L –1), 像水性 Fe, 沿着流动路径浓度下降。X 射线吸收光谱表明,Fe、As 和 SO 4 2–水溶液中的下游衰减是由于无定形砷酸铁 (AFA)、施韦特曼石和黄钾铁矾的沉淀造成的。这些固体中的 Fe(III) 在 δ 56 Fe 中表现出极大的可变性,从 AMD 源附近的 AFA 中的 +3.95‰ 到下游约 450 m 的施韦特曼石中的 -1.34‰。类似地,固体 Fe(III) 沉淀物和水性 Fe 之间的同位素对比 (Δ 56 Fe ppt-aq ) 沿着流动路径从大约 +4.1 下降到 −1.1‰。从正到负的转变 Δ 56 Fe ppt-aq反映了竞争平衡与动力学分馏过程之间的差异。
更新日期:2022-11-28
中文翻译:

酸性矿山排水中的铁同位素:固体(Schwertmannite、Jarosite 和砷酸铁)和水相之间的极端和发散分馏
检查稳定的 Fe 同位素是探索各种环境中 Fe 循环的有力工具。然而,酸性矿山废水 (AMD) 中 Fe 的同位素分馏很少受到关注,也知之甚少。在这里,我们分析了沿 AMD 流路的水域和富含 Fe(III) 的固体中的 Fe 同位素。含水 Fe 的浓度和 δ 56 Fe 范围从 AMD 源处的 ∼420 mg L –1和 + 0.04‰ 到下游 ∼450 m 处的∼100 mg L –1 和 −0.81‰ 。As 水溶液(高达 ∼33 mg L –1)和 SO 4 2–(高达 ∼2000 mg L –1), 像水性 Fe, 沿着流动路径浓度下降。X 射线吸收光谱表明,Fe、As 和 SO 4 2–水溶液中的下游衰减是由于无定形砷酸铁 (AFA)、施韦特曼石和黄钾铁矾的沉淀造成的。这些固体中的 Fe(III) 在 δ 56 Fe 中表现出极大的可变性,从 AMD 源附近的 AFA 中的 +3.95‰ 到下游约 450 m 的施韦特曼石中的 -1.34‰。类似地,固体 Fe(III) 沉淀物和水性 Fe 之间的同位素对比 (Δ 56 Fe ppt-aq ) 沿着流动路径从大约 +4.1 下降到 −1.1‰。从正到负的转变 Δ 56 Fe ppt-aq反映了竞争平衡与动力学分馏过程之间的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号