当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncovering the Electrolyte-Dependent Transport Mechanism of LiO2 in Lithium-Oxygen Batteries
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-28 , DOI: 10.1021/jacs.2c09700 Zhen Jiang 1 , Andrew M Rappe 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-28 , DOI: 10.1021/jacs.2c09700 Zhen Jiang 1 , Andrew M Rappe 1
Affiliation
|
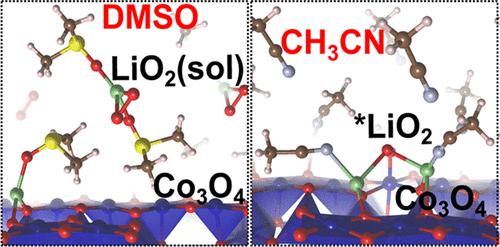
Lithium-oxygen batteries (LOBs) offer extremely high theoretical energy density and are therefore strong contenders for bringing conventional batteries into the next generation. To avoid deactivation and passivation of the electrode due to the gradual covering of the surface by discharge products, electrolytes with high donor number (DN) are becoming increasingly popular in LOBs. However, the mechanism of this electrolyte-assisted discharge process remains unclear in many aspects, including the lithium superoxide (LiO2) intermediate transportation mechanism and stability at both electrode/electrolyte interfaces and in bulk electrolytes. Here, we performed a systematic Born–Oppenheimer molecular dynamics (BOMD)-level investigation of the LiO2 solvation reactions at two interfaces with high- or low-DN electrolytes (dimethyl sulfoxide (DMSO) or acetonitrile (CH3CN), respectively), followed by examinations of stability and condensation once the LiO2 monomers are solvated. Release of partial discharge product LiO2 is found to be energetically favorable into DMSO from the Co3O4 cathode with a small energy barrier. However, in the presence of CH3CN electrolyte, the release of LiO2 from the electrode surface is found to be energetically unfavorable. Dissolved LiO2(sol) clusters in bulk DMSO solvents are found to be more favorable to dimerize and agglomerate into a toroidal shape rather than to decompose, which avoids the emergence of strong oxidant ions (O2–) and preserves the system stability. This study provides two complete molecular-level pathways (solution and surface) from first-principles understanding of LOBs, offering guidance for future selection and design of electrode catalysts and solvents.
中文翻译:

揭示锂氧电池中 LiO2 依赖于电解质的传输机制
锂氧电池 (LOB) 具有极高的理论能量密度,因此是将传统电池带入下一代的有力竞争者。为了避免由于放电产物逐渐覆盖表面而导致电极失活和钝化,具有高供体数 (DN) 的电解质在 LOB 中越来越受欢迎。然而,这种电解质辅助放电过程的机制在许多方面仍不清楚,包括超氧化锂 (LiO 2 ) 中间传输机制以及电极/电解质界面和本体电解质中的稳定性。在这里,我们对 LiO 2进行了系统的玻恩-奥本海默分子动力学 (BOMD) 级研究在高 DN 或低 DN 电解质(分别为二甲基亚砜 (DMSO) 或乙腈 (CH 3 CN))的两个界面处进行溶剂化反应,然后在 LiO 2单体溶剂化后检查稳定性和缩合。发现局部放电产物 LiO 2的释放在能量上有利于从具有小能垒的 Co 3 O 4阴极进入 DMSO。然而,在存在 CH 3 CN 电解质的情况下,发现 LiO 2从电极表面的释放在能量上是不利的。溶解的LiO 2研究发现,本体 DMSO 溶剂中的 (sol) 团簇更有利于二聚和团聚成环形而不是分解,这避免了强氧化剂离子 (O 2 – ) 的出现并保持了系统稳定性。本研究从对 LOB 的第一性原理理解提供了两个完整的分子水平途径(溶液和表面),为未来电极催化剂和溶剂的选择和设计提供了指导。
更新日期:2022-11-28
中文翻译:

揭示锂氧电池中 LiO2 依赖于电解质的传输机制
锂氧电池 (LOB) 具有极高的理论能量密度,因此是将传统电池带入下一代的有力竞争者。为了避免由于放电产物逐渐覆盖表面而导致电极失活和钝化,具有高供体数 (DN) 的电解质在 LOB 中越来越受欢迎。然而,这种电解质辅助放电过程的机制在许多方面仍不清楚,包括超氧化锂 (LiO 2 ) 中间传输机制以及电极/电解质界面和本体电解质中的稳定性。在这里,我们对 LiO 2进行了系统的玻恩-奥本海默分子动力学 (BOMD) 级研究在高 DN 或低 DN 电解质(分别为二甲基亚砜 (DMSO) 或乙腈 (CH 3 CN))的两个界面处进行溶剂化反应,然后在 LiO 2单体溶剂化后检查稳定性和缩合。发现局部放电产物 LiO 2的释放在能量上有利于从具有小能垒的 Co 3 O 4阴极进入 DMSO。然而,在存在 CH 3 CN 电解质的情况下,发现 LiO 2从电极表面的释放在能量上是不利的。溶解的LiO 2研究发现,本体 DMSO 溶剂中的 (sol) 团簇更有利于二聚和团聚成环形而不是分解,这避免了强氧化剂离子 (O 2 – ) 的出现并保持了系统稳定性。本研究从对 LOB 的第一性原理理解提供了两个完整的分子水平途径(溶液和表面),为未来电极催化剂和溶剂的选择和设计提供了指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号