当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strategies for Assessing Acceptable Intakes for Novel N-Nitrosamines Derived from Active Pharmaceutical Ingredients
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.jmedchem.2c01498 David J Ponting 1 , Krista L Dobo 2 , Michelle O Kenyon 2 , Amit S Kalgutkar 3
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-11-28 , DOI: 10.1021/acs.jmedchem.2c01498 David J Ponting 1 , Krista L Dobo 2 , Michelle O Kenyon 2 , Amit S Kalgutkar 3
Affiliation
|
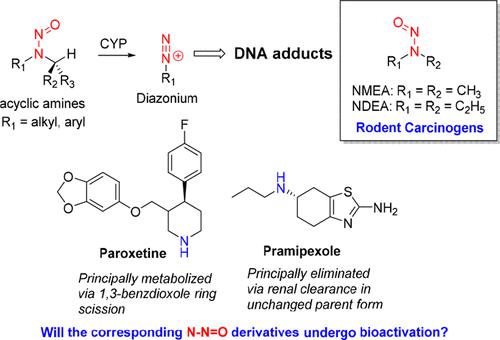
The detection of N-nitrosamines, derived from solvents and reagents and, on occasion, the active pharmaceutical ingredient (API) at higher than acceptable levels in drug products, has led regulators to request a detailed review for their presence in all medicinal products. In the absence of rodent carcinogenicity data for novel N-nitrosamines derived from amine-containing APIs, a conservative class limit of 18 ng/day (based on the most carcinogenic N-nitrosamines) or the derivation of acceptable intakes (AIs) using structurally related surrogates with robust rodent carcinogenicity data is recommended. The guidance has implications for the pharmaceutical industry given the vast number of marketed amine-containing drugs. In this perspective, the rate-limiting step in N-nitrosamine carcinogenicity, involving cytochrome P450-mediated α-carbon hydroxylation to yield DNA-reactive diazonium or carbonium ion intermediates, is discussed with reference to the selection of read-across analogs to derive AIs. Risk-mitigation strategies for managing putative N-nitrosamines in the preclinical discovery setting are also presented.
中文翻译:

评估源自活性药物成分的新型 N-亚硝胺可接受摄入量的策略
检测到来自溶剂和试剂的N-亚硝胺,有时还检测到药品中活性药物成分 (API) 的含量高于可接受的水平,导致监管机构要求对其在所有医药产品中的存在情况进行详细审查。在缺乏源自含胺 API的新型N-亚硝胺的啮齿动物致癌性数据的情况下,保守的类别限值为 18 ng/天(基于最致癌的N-nitrosamines) 或使用具有可靠啮齿动物致癌性数据的结构相关替代物推导可接受摄入量 (AI)。鉴于市售的含胺药物数量巨大,该指南对制药行业具有重要意义。从这个角度来看, N-亚硝胺致癌性的限速步骤,涉及细胞色素 P450 介导的 α-碳羟基化产生 DNA 反应性重氮或碳正离子中间体,参考交叉读取类似物的选择以推导 AI 进行讨论. 还介绍了在临床前发现环境中管理假定的N -亚硝胺的风险缓解策略。
更新日期:2022-11-28
中文翻译:

评估源自活性药物成分的新型 N-亚硝胺可接受摄入量的策略
检测到来自溶剂和试剂的N-亚硝胺,有时还检测到药品中活性药物成分 (API) 的含量高于可接受的水平,导致监管机构要求对其在所有医药产品中的存在情况进行详细审查。在缺乏源自含胺 API的新型N-亚硝胺的啮齿动物致癌性数据的情况下,保守的类别限值为 18 ng/天(基于最致癌的N-nitrosamines) 或使用具有可靠啮齿动物致癌性数据的结构相关替代物推导可接受摄入量 (AI)。鉴于市售的含胺药物数量巨大,该指南对制药行业具有重要意义。从这个角度来看, N-亚硝胺致癌性的限速步骤,涉及细胞色素 P450 介导的 α-碳羟基化产生 DNA 反应性重氮或碳正离子中间体,参考交叉读取类似物的选择以推导 AI 进行讨论. 还介绍了在临床前发现环境中管理假定的N -亚硝胺的风险缓解策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号