当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ambimodal Bispericyclic [6 + 4]/[4 + 6] Transition State Competes with Diradical Pathways in the Cycloheptatriene Dimerization: Dynamics and Experimental Characterization of Thermal Dimers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-28 , DOI: 10.1021/jacs.2c10407 Qingyang Zhou 1 , Mathias K Thøgersen 2 , Nomaan M Rezayee 2 , Karl Anker Jørgensen 2 , K N Houk 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-11-28 , DOI: 10.1021/jacs.2c10407 Qingyang Zhou 1 , Mathias K Thøgersen 2 , Nomaan M Rezayee 2 , Karl Anker Jørgensen 2 , K N Houk 3
Affiliation
|
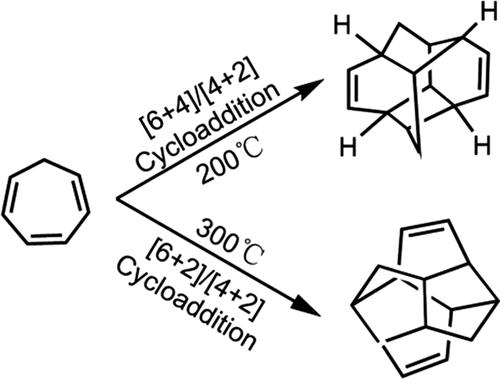
The thermal dimerization of cycloheptatriene is predicted to occur by a concerted [6 + 4] cycloaddition via an ambimodal [6 + 4]/[4 + 6] transition state (TS) and a competing stepwise diradical (6 + 2) cycloaddition; both dimers subsequently undergo intramolecular [4 + 2] cycloadditions to afford thermally stable tetracyclic products. The ambimodal TS is the 10π-electron version of the prototype bispericyclic dimerization of cyclopentadiene discovered by Caramella et al. in 2002. Quantum mechanical studies using several common DFT functionals and post-HF methods, ωB97X-D, M06-2X, DLPNO-CCSD(T), NEVPT2, and PWPB95-D3(BJ), and quasiclassical molecular dynamics simulations provide details of bond timing and bifurcation pathways. By comparing the ambimodal [6 + 4]/[4 + 6] TS for cycloheptatriene dimerization with the ambimodal [4 + 2]/[2 + 4] TS of cyclopentadiene dimerization, we found that the high distortion energy in cycloheptatriene dimerization is the key to its relatively high energy barrier. The computational investigations were coupled with experimental studies of the cycloheptatriene dimerization, which resulted in the isolation of the two tetracyclic dimers. At lower temperature, the product from the predicted exo-[6 + 4]/[4 + 6] cycloaddition, followed by a subsequent intramolecular [4 + 2] cycloaddition, predominantly forms, while at higher temperature, the diradical (6 + 2) cycloadduct is the major product.
中文翻译:

双峰双周环 [6 + 4]/[4 + 6] 过渡态与环庚三烯二聚化中的双自由基途径竞争:热二聚体的动力学和实验表征
预计环庚三烯的热二聚会通过双峰 [6 + 4]/[4 + 6] 过渡态 (TS) 和竞争逐步双自由基 (6 + 2) 环加成的协同 [6 + 4] 环加成发生;两种二聚体随后都进行分子内 [4 + 2] 环加成,得到热稳定的四环产物。双峰 TS 是 Caramella等人发现的环戊二烯原型双周环二聚化的 10π 电子版本。2002 年。使用几种常见的 DFT 泛函和后 HF 方法的量子力学研究,ωB97X-D、M06-2X、DLPNO-CCSD(T)、NEVPT2 和 PWPB95-D3(BJ),以及准经典分子动力学模拟提供了详细信息债券时间和分叉路径。通过比较环庚三烯二聚的双峰[6 + 4] / [4 + 6] TS与环戊二烯二聚的双峰[4 + 2] / [2 + 4] TS,我们发现环庚三烯二聚中的高畸变能量是其相对较高的能垒的关键。计算研究与环庚三烯二聚化的实验研究相结合,导致两个四环二聚体的分离。在较低的温度下,预测的exo的产物-[6 + 4]/[4 + 6] 环加成,随后是随后的分子内 [4 + 2] 环加成,主要形成,而在较高温度下,双自由基 (6 + 2) 环加合物是主要产物。
更新日期:2022-11-28
中文翻译:

双峰双周环 [6 + 4]/[4 + 6] 过渡态与环庚三烯二聚化中的双自由基途径竞争:热二聚体的动力学和实验表征
预计环庚三烯的热二聚会通过双峰 [6 + 4]/[4 + 6] 过渡态 (TS) 和竞争逐步双自由基 (6 + 2) 环加成的协同 [6 + 4] 环加成发生;两种二聚体随后都进行分子内 [4 + 2] 环加成,得到热稳定的四环产物。双峰 TS 是 Caramella等人发现的环戊二烯原型双周环二聚化的 10π 电子版本。2002 年。使用几种常见的 DFT 泛函和后 HF 方法的量子力学研究,ωB97X-D、M06-2X、DLPNO-CCSD(T)、NEVPT2 和 PWPB95-D3(BJ),以及准经典分子动力学模拟提供了详细信息债券时间和分叉路径。通过比较环庚三烯二聚的双峰[6 + 4] / [4 + 6] TS与环戊二烯二聚的双峰[4 + 2] / [2 + 4] TS,我们发现环庚三烯二聚中的高畸变能量是其相对较高的能垒的关键。计算研究与环庚三烯二聚化的实验研究相结合,导致两个四环二聚体的分离。在较低的温度下,预测的exo的产物-[6 + 4]/[4 + 6] 环加成,随后是随后的分子内 [4 + 2] 环加成,主要形成,而在较高温度下,双自由基 (6 + 2) 环加合物是主要产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号