European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-11-24 , DOI: 10.1016/j.ejmech.2022.114909 Marian N Aziz 1 , Linh Nguyen 2 , Yan Chang 3 , Delphine Gout 4 , Zui Pan 3 , Carl J Lovely 4
|
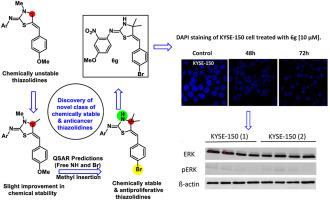
The discovery of a new class of extracellular-signal-regulated kinase (ERK) inhibitors has been achieved via developing novel 2-imino-5-arylidene-thiazolidine analogues. A novel synthetic method employing a solid support-mediated reaction was used to construct the targeted thiazolidines through a cascade reaction with good yields. The chemical and physical stability of the new thiazolidine library has successfully been achieved by blocking the labile C5-position to aerobic oxidation. A cell viability study was performed using esophageal squamous cell carcinoma cell lines (KYSE-30 and KYSE-150) and non-tumorous esophageal epithelial cell lines (HET-1A and NES-G4T) through utilization of an MTT assay, revealing that (Z)-5-((Z)-4-bromobenzylidene)-N-(4-methoxy-2-nitrophenyl)-4,4-dimethylthiazolidin-2-imine (6g) was the best compound among the synthesized library in terms of selectivity. DAPI staining experiments were performed to visualize the morphological changes and to investigate the apoptotic activity. Moreover, western blots were used to probe the mechanism/pathway behind the observed activity/selectivity of thiazolidine 6g which established selective inhibition of phosphorylation in the ERK pathway. Molecular modeling techniques have been utilized to confirm the observed activity. A molecular docking study revealed similar binding interactions between the synthesized thiazolidines and reported co-crystalized inhibitors with ERK proteins. Thus, the present study provides a starting point for the development of interesting bioactive 2-imino-5-arylidene-thiazolidines.
中文翻译:

通过 ERK 途径对食管鳞状细胞癌具有潜在抗增殖特性的新型噻唑烷
通过开发新型 2-imino-5-arylidene-thiazolidine 类似物,已经发现了一类新的细胞外信号调节激酶 (ERK) 抑制剂。一种采用固体载体介导反应的新型合成方法通过级联反应以良好的产率构建了靶向噻唑烷。新型噻唑烷库的化学和物理稳定性已通过阻断不稳定的 C5 位以进行有氧氧化而成功实现。通过使用 MTT 测定法,使用食管鳞状细胞癌细胞系(KYSE-30 和 KYSE-150)和非肿瘤性食管上皮细胞系(HET-1A 和 NES-G4T)进行细胞活力研究,结果表明 ( Z )-5-(( Z )-4-溴亚苄基)- N-(4-methoxy-2-nitrophenyl)-4,4-dimethylthiazolidin-2-imine ( 6g ) 是合成库中选择性最好的化合物。进行 DAPI 染色实验以可视化形态变化并研究细胞凋亡活性。此外,western blots 被用来探索所观察到的噻唑烷6g的活性/选择性背后的机制/途径这建立了对 ERK 通路中磷酸化的选择性抑制。分子建模技术已被用于确认观察到的活性。分子对接研究揭示了合成的噻唑烷与报道的与 ERK 蛋白共结晶的抑制剂之间存在相似的结合相互作用。因此,本研究为开发有趣的生物活性 2-亚氨基-5-亚芳基-噻唑烷提供了一个起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号