当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 3-Aryl Oxindoles having Trifluoromethylated Quaternary Stereocenters via Catalyst Free Arylation
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-24 , DOI: 10.1002/adsc.202200918 Chavakula Nagababu 1 , Jaggaraju Prudhviraj 1 , Punna Nagender 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-24 , DOI: 10.1002/adsc.202200918 Chavakula Nagababu 1 , Jaggaraju Prudhviraj 1 , Punna Nagender 1
Affiliation
|
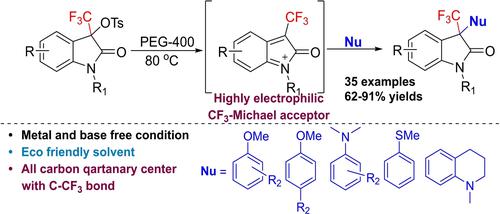
Herein we report the synthesis of 3-aryl oxindoles having trifluoromethylated quaternary stereocenters through the catalyst free arylation of 3-trifluoromethyl,3-tosyloxy oxindoles with anisoles. The transformation proceeds via electrophilic 3-(trifluoromethyl)-2H-indolium intermediate (Michael acceptor), which is generated by the heterolytic cleavage of C-OTs bond under metal-free conditions in PEG-400. The developed strategy offers broad substrate scope, extending to N,N-dimethyl-anilines, and thio-anisole to afford the range of 3-trifluoromethyl,3-aryl oxindoles in 62–91% yields.
中文翻译:

无催化剂芳基化合成具有三氟甲基化季立体中心的 3-芳基羟吲哚
在此,我们报告了通过 3-三氟甲基,3-甲苯磺酰氧基羟吲哚与苯甲醚的无催化剂芳基化反应,合成了具有三氟甲基化季立体中心的 3-芳基羟吲哚。转化通过亲电子 3-(三氟甲基)-2 H-吲哚中间体(Michael 受体)进行,该中间体由 C-OTs 键在 PEG-400 中无金属条件下异裂解产生。开发的策略提供了广泛的底物范围,扩展到N,N-二甲基苯胺和硫代苯甲醚,以 62-91% 的收率提供 3-三氟甲基、3-芳基羟吲哚的范围。
更新日期:2022-11-24
中文翻译:

无催化剂芳基化合成具有三氟甲基化季立体中心的 3-芳基羟吲哚
在此,我们报告了通过 3-三氟甲基,3-甲苯磺酰氧基羟吲哚与苯甲醚的无催化剂芳基化反应,合成了具有三氟甲基化季立体中心的 3-芳基羟吲哚。转化通过亲电子 3-(三氟甲基)-2 H-吲哚中间体(Michael 受体)进行,该中间体由 C-OTs 键在 PEG-400 中无金属条件下异裂解产生。开发的策略提供了广泛的底物范围,扩展到N,N-二甲基苯胺和硫代苯甲醚,以 62-91% 的收率提供 3-三氟甲基、3-芳基羟吲哚的范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号