当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereoselective Radical Aminoacylation of Olefins through N-Heterocyclic Carbene Catalysis
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-11-24 , DOI: 10.1021/jacs.2c11209 Wen-Deng Liu 1 , Woojin Lee 2 , Hanyu Shu 1 , Chuyu Xiao 1 , Huiwei Xu 1 , Xiangyang Chen 2 , Kendall N Houk 2 , Jiannan Zhao 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-11-24 , DOI: 10.1021/jacs.2c11209 Wen-Deng Liu 1 , Woojin Lee 2 , Hanyu Shu 1 , Chuyu Xiao 1 , Huiwei Xu 1 , Xiangyang Chen 2 , Kendall N Houk 2 , Jiannan Zhao 1
Affiliation
|
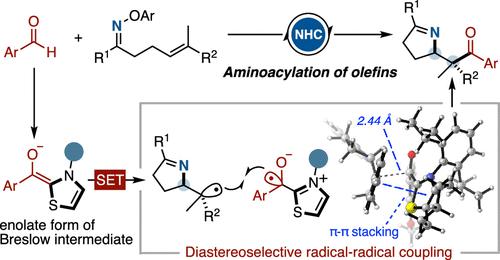
There have been significant advancements in radical-mediated reactions through covalent-based organocatalysis. Here, we present the generation of iminyl and amidyl radicals via N-heterocyclic carbene (NHC) catalysis, enabling diastereoselective aminoacylation of trisubstituted alkenes. Different from photoredox catalysis, single electron transfer from the deprotonated Breslow intermediate to O-aryl hydroxylamine generates an NHC-bound ketyl radical, which undergoes diastereocontrolled cross-coupling with the prochiral C-centered radical. This operationally simple method provides a straightforward access to a variety of pyrroline and oxazolidinone heterocycles with vicinal stereocenters (77 examples, up to >19:1 d.r.). Electrochemical studies of the acyl thiazolium salts support our reaction design and highlight the reducing ability of Breslow-type derivatives. A detailed computational analysis of this organocatalytic system suggests that radical–radical coupling is the rate-determining step, in which π–π stacking interaction between the radical intermediates subtly controls the diastereoselectivity.
中文翻译:

N-杂环卡宾催化烯烃的非对映选择性自由基氨酰化
通过基于共价键的有机催化,自由基介导的反应取得了重大进展。在这里,我们介绍了通过 N-杂环卡宾 (NHC) 催化生成亚胺基和酰胺基自由基,从而实现三取代烯烃的非对映选择性氨基酰化。与光氧化还原催化不同,单电子从去质子化的 Breslow 中间体转移到O-芳基羟胺生成 NHC 结合的羰基自由基,该自由基与前手性 C 中心自由基发生非对映控制的交叉偶联。这种操作简单的方法可以直接获取各种具有邻位立体中心的吡咯啉和恶唑烷酮杂环化合物(77 个实例,高达 >19:1 dr)。酰基噻唑盐的电化学研究支持我们的反应设计并突出了 Breslow 型衍生物的还原能力。对该有机催化系统的详细计算分析表明,自由基-自由基偶联是决速步骤,其中自由基中间体之间的 π-π 堆积相互作用巧妙地控制了非对映选择性。
更新日期:2022-11-24
中文翻译:

N-杂环卡宾催化烯烃的非对映选择性自由基氨酰化
通过基于共价键的有机催化,自由基介导的反应取得了重大进展。在这里,我们介绍了通过 N-杂环卡宾 (NHC) 催化生成亚胺基和酰胺基自由基,从而实现三取代烯烃的非对映选择性氨基酰化。与光氧化还原催化不同,单电子从去质子化的 Breslow 中间体转移到O-芳基羟胺生成 NHC 结合的羰基自由基,该自由基与前手性 C 中心自由基发生非对映控制的交叉偶联。这种操作简单的方法可以直接获取各种具有邻位立体中心的吡咯啉和恶唑烷酮杂环化合物(77 个实例,高达 >19:1 dr)。酰基噻唑盐的电化学研究支持我们的反应设计并突出了 Breslow 型衍生物的还原能力。对该有机催化系统的详细计算分析表明,自由基-自由基偶联是决速步骤,其中自由基中间体之间的 π-π 堆积相互作用巧妙地控制了非对映选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号