当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Density Functional Theory Study on [Ni0(1,10-Phenanthroline)]-Catalyzed Reductive Carboxylation of Alkyl and Aryl Halides with CO2: Effect of the Lewis Acid and β-H Elimination Side Reaction in the Crucial CO2 Insertion Step
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-11-22 , DOI: 10.1021/acs.inorgchem.2c03340 Prabha Vadivelu 1 , Krithika Ganesan 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-11-22 , DOI: 10.1021/acs.inorgchem.2c03340 Prabha Vadivelu 1 , Krithika Ganesan 1
Affiliation
|
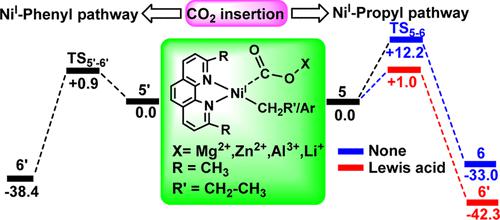
The [Ni0(phen)] (1)-catalyzed reductive carboxylation of propyl and phenyl chloride with a CO2 molecule has been compared using the density functional theory method. The reactivity of 1 in the initial oxidative addition with the propyl and phenyl chloride and in the subsequent single electron transfer step to form the NiI intermediates, [NiI(phen)(R′-CH2)], 4, (R′ = CH3–CH2−), and [NiI(phen)(C6H5)], 4′, is almost the same. However, an apparent reactivity difference is interpreted in the CO2 insertion step, which involves the NiI and the NiII intermediates. In both propyl and phenyl chloride, the NiI-mediated CO2 insertion is kinetically more preferred than that mediated by the analogues NiII intermediates (3 and 3′) by +5.0 and +33.4 kcal/mol, respectively. This trend in energetics clearly shows that the CO2 insertion of phenyl chloride exclusively occurs via the NiI-mediated pathway, whereas in propyl chloride, it follows both NiI- and NiII-mediated pathways. Overall, the catalytic efficacy of 1 is found to be higher in phenyl chloride (by +11.3 kcal/mol) than that in propyl chloride. Furthermore, the effect of a plausible β-H elimination side reaction in the CO2 insertion step is modeled for propyl chloride. Herein, the β-H elimination of the NiII propyl species (3) is kinetically more feasible than its CO2 insertion, while the β-H elimination of NiI propyl (4) is rather difficult compared to its CO2 insertion by +16.3 kcal/mol. This strongly supports the suitability of the NiI intermediate in the CO2 insertion step. In addition, the role of the Lewis acid (X) in the CO2 insertion step is tested by incorporating various Lewis acids (X = MgCl2, ZnCl2, AlCl3, and LiCl) in the NiII propyl (7) and NiI propyl (5) intermediates. The Lewis acids effectively facilitate the CO2 insertion step, and the effect due to MgCl2 is found to be more evident. MgCl2 enhances the CO2 insertion of 5 and 7 by 89 and 84%, respectively, and hence, the NiI-mediated CO2 insertion of propyl halide (ΔG⧧ = +1.4 kcal/mol) is now comparable with that of phenyl halide (ΔG⧧ = +0.9 kcal/mol). This suggests that in the presence of Lewis acids, the catalytic efficacy of 1 is enhanced for the reductive carboxylation of propyl halide and exhibits similar reactivity to that of phenyl halide.
中文翻译:

[Ni0(1,10-菲咯啉)]-催化烷基和芳基卤化物与 CO2 的还原羧基化的密度泛函理论研究:关键 CO2 插入步骤中路易斯酸和 β-H 消除副反应的影响
使用密度泛函理论方法比较了[Ni 0 (phen)] ( 1 )-催化的丙基和苯基氯与CO 2分子的还原羧化。1在与丙基氯和苯氯的初始氧化加成反应以及随后的单电子转移步骤中形成 Ni I中间体[Ni I (phen)(R'-CH 2 )], 4 , (R' = CH 3 –CH 2 −), 和 [Ni I (phen)(C 6 H 5 )], 4′,几乎是一样的。然而,在涉及 Ni I和 Ni II中间体的 CO 2插入步骤中解释了明显的反应性差异。在丙基氯和苯基氯中,Ni I介导的 CO 2插入在动力学上比由类似物 Ni II中间体(3和3' )介导的插入更优选,分别为 +5.0 和 +33.4 kcal/mol。这种能量学趋势清楚地表明,苯基氯的 CO 2插入完全通过 Ni I介导的途径发生,而在丙基氯中,它遵循 Ni I - 和 NiII介导的途径。总体而言,发现1在苯基氯中的催化功效(+11.3 kcal/mol)高于丙基氯。此外,针对丙基氯模拟了CO 2插入步骤中可能发生的 β-H 消除副反应的影响。在此,Ni II丙基物质 ( 3 )的 β-H 消除在动力学上比其 CO 2插入更可行,而 Ni I丙基 ( 4 )的 β-H 消除与其 CO 2插入相比通过+ 16.3 大卡/摩尔。这强烈支持 Ni I中间体在 CO 2中的适用性插入步骤。此外,路易斯酸 (X) 在 CO 2插入步骤中的作用通过在 Ni II丙基 ( 7 ) 和 Ni中加入各种路易斯酸(X = MgCl 2、ZnCl 2、AlCl 3和 LiCl)来测试一丙基(5)中间体。路易斯酸有效地促进了CO 2插入步骤,并且发现由于MgCl 2引起的效果更加明显。MgCl 2分别将5和7的 CO 2插入增强了89% 和 84%,因此,Ni I介导的 CO2丙基卤化物 (Δ G ⧧ = +1.4 kcal/mol) 的插入现在与苯卤化物 (Δ G ⧧ = +0.9 kcal/mol) 相当。这表明在路易斯酸存在的情况下,1对卤代丙烷的还原羧化作用的催化效果增强,并表现出与卤代苯相似的反应活性。
更新日期:2022-11-22
中文翻译:

[Ni0(1,10-菲咯啉)]-催化烷基和芳基卤化物与 CO2 的还原羧基化的密度泛函理论研究:关键 CO2 插入步骤中路易斯酸和 β-H 消除副反应的影响
使用密度泛函理论方法比较了[Ni 0 (phen)] ( 1 )-催化的丙基和苯基氯与CO 2分子的还原羧化。1在与丙基氯和苯氯的初始氧化加成反应以及随后的单电子转移步骤中形成 Ni I中间体[Ni I (phen)(R'-CH 2 )], 4 , (R' = CH 3 –CH 2 −), 和 [Ni I (phen)(C 6 H 5 )], 4′,几乎是一样的。然而,在涉及 Ni I和 Ni II中间体的 CO 2插入步骤中解释了明显的反应性差异。在丙基氯和苯基氯中,Ni I介导的 CO 2插入在动力学上比由类似物 Ni II中间体(3和3' )介导的插入更优选,分别为 +5.0 和 +33.4 kcal/mol。这种能量学趋势清楚地表明,苯基氯的 CO 2插入完全通过 Ni I介导的途径发生,而在丙基氯中,它遵循 Ni I - 和 NiII介导的途径。总体而言,发现1在苯基氯中的催化功效(+11.3 kcal/mol)高于丙基氯。此外,针对丙基氯模拟了CO 2插入步骤中可能发生的 β-H 消除副反应的影响。在此,Ni II丙基物质 ( 3 )的 β-H 消除在动力学上比其 CO 2插入更可行,而 Ni I丙基 ( 4 )的 β-H 消除与其 CO 2插入相比通过+ 16.3 大卡/摩尔。这强烈支持 Ni I中间体在 CO 2中的适用性插入步骤。此外,路易斯酸 (X) 在 CO 2插入步骤中的作用通过在 Ni II丙基 ( 7 ) 和 Ni中加入各种路易斯酸(X = MgCl 2、ZnCl 2、AlCl 3和 LiCl)来测试一丙基(5)中间体。路易斯酸有效地促进了CO 2插入步骤,并且发现由于MgCl 2引起的效果更加明显。MgCl 2分别将5和7的 CO 2插入增强了89% 和 84%,因此,Ni I介导的 CO2丙基卤化物 (Δ G ⧧ = +1.4 kcal/mol) 的插入现在与苯卤化物 (Δ G ⧧ = +0.9 kcal/mol) 相当。这表明在路易斯酸存在的情况下,1对卤代丙烷的还原羧化作用的催化效果增强,并表现出与卤代苯相似的反应活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号