Analytica Chimica Acta ( IF 6.2 ) Pub Date : 2022-11-21 , DOI: 10.1016/j.aca.2022.340650 Chih-Ning Cheng , Yu-Fong Peng , Ju-Yu Chen , Guan-Yuan Chen , Te-I Weng , Ching-Hua Kuo
|
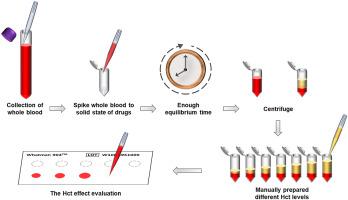
The application of dried blood spots (DBS) has gradually increased in different fields because of its several advantages. The hematocrit (Hct) effect is one major analytical challenge that may affect the quantification accuracy of DBS samples and should be investigated when developing a novel DBS method. However, previous studies usually overlooked the Hct-related distribution bias when evaluating the Hct effect. This study aimed to propose an effective DBS preparation protocol for the comprehensive evaluation of the Hct effect. We selected voriconazole and posaconazole as the demonstration drugs. Fifteen microliters of the blood samples were spotted on DBS cards followed by whole spot extraction. An LC-MS/MS method was first developed to quantify voriconazole and posaconazole in DBS samples. The quantitation accuracy for both azole drugs was within 93.5%–111.7%, except for the accuracies of posaconazole at the LLOQ, which were less than 119%. The intra- and interday precision were below 11%. The validated LC-MS/MS method was used to develop the DBS preparation protocol for evaluating the Hct effect. Three critical parameters that may affect the observed Hct effect were investigated. The results showed that using the solid-state of the target analytes, spiking the target analytes before preparing different Hct levels, and allowing enough equilibrium time after spiking target analytes can provide a more holistic Hct effect evaluation. The validity of the proposed new protocol was verified by conversion factors obtained from 71 paired DBS and plasma samples. Conversion factors calculated by clinical samples were consistent with the Hct effect evaluated by manually prepared DBS samples. This new DBS preparation protocol eliminated the common pitfalls in studying the Hct effect and offered a comprehensive strategy to assess the Hct effect for further DBS studies.
中文翻译:

用于综合评价血细胞比容效应的干血斑制备方案的开发
干血斑 (DBS) 的应用由于其诸多优点在不同领域逐渐增加。血细胞比容 (Hct) 效应是一个主要的分析挑战,可能会影响 DBS 样品的定量准确性,在开发新的 DBS 方法时应加以研究。然而,以往的研究在评估Hct效应时,往往忽略了Hct相关的分布偏倚。本研究旨在提出一种有效的 DBS 制备方案,用于综合评估 Hct 效应。我们选择伏立康唑和泊沙康唑作为示范药物。在 DBS 卡上点样 15 微升血样,然后进行全点提取。首先开发了一种 LC-MS/MS 方法来定量 DBS 样品中的伏立康唑和泊沙康唑。两种唑类药物的定量准确度在 93.5%–111.7% 之间,但泊沙康唑在 LLOQ 的准确度低于 119%。日内和日间精确度均低于 11%。经过验证的 LC-MS/MS 方法用于开发用于评估 Hct 效果的 DBS 制备方案。研究了可能影响观察到的 Hct 效应的三个关键参数。结果表明,利用目标分析物的固态,在制备不同水平的 Hct 之前加标目标分析物,并在加标目标分析物后留出足够的平衡时间,可以提供更全面的 Hct 效果评估。通过从 71 个配对的 DBS 和血浆样本中获得的转换因子验证了所提出的新协议的有效性。临床样本计算的转换因子与手动制备的 DBS 样本评估的 Hct 效应一致。这种新的 DBS 制备方案消除了研究 Hct 效应的常见缺陷,并提供了一个全面的策略来评估 Hct 效应以供进一步的 DBS 研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号