当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The metal dependence of single-metal mediated phosphodiester bond cleavage: a QM/MM study of a multifaceted human enzyme
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-11-21 , DOI: 10.1039/d2cp04338f Rajwinder Kaur 1 , Mohamed M Aboelnga 1 , Dylan J Nikkel 1 , Stacey D Wetmore 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-11-21 , DOI: 10.1039/d2cp04338f Rajwinder Kaur 1 , Mohamed M Aboelnga 1 , Dylan J Nikkel 1 , Stacey D Wetmore 1
Affiliation
|
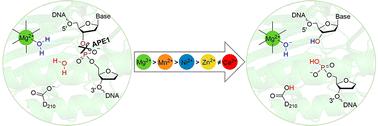
Nucleases catalyze the cleavage of phosphodiester bonds in nucleic acids using a range of metal cofactors. Although it is well accepted that many nucleases rely on two metal ions, the one-metal mediated pathway is debated. Furthermore, one-metal mediated nucleases maintain activity in the presence of many different metals, but the underlying reasons for this broad metal specificity are unknown. The human apurinic/apyrimidinic endonuclease (APE1), which plays a key role in DNA repair, transcription regulation, and gene expression, is a prototypical example of a one-metal dependent nuclease. Although Mg2+ is the native metal cofactor, APE1 remains catalytically active in the presence of several metals, with the rate decreasing as Mg2+ > Mn2+ > Ni2+ > Zn2+, while Ca2+ completely abolished the activity. The present work uses quantum mechanics–molecular mechanics techniques to map APE1-facilitated phosphodiester bond hydrolysis in the presence of these metals. The structural differences in stationary points along the reaction pathway shed light on the interplay between several factors that allow APE1 to remain catalytically active for various metals, with the trend in the barrier heights correlating with the experimentally reported APE1 catalytic activity. In contrast, Ca2+ significantly changes the metal coordination and active site geometry, and thus completely inhibits catalysis. Our work thereby provides support for the controversial single-metal mediated phosphodiester bond cleavage and clarifies uncertainties regarding the role of the metal and metal identity in this important reaction. This information is key for future medicinal and biotechnological applications including disease diagnosis and treatment, and protein engineering.
中文翻译:

单金属介导的磷酸二酯键断裂的金属依赖性:多方面人类酶的 QM/MM 研究
核酸酶使用一系列金属辅因子催化核酸中磷酸二酯键的裂解。尽管人们普遍认为许多核酸酶依赖于两种金属离子,但单金属介导的途径仍有争议。此外,单金属介导的核酸酶在许多不同金属存在的情况下保持活性,但这种广泛的金属特异性的潜在原因尚不清楚。人类脱嘌呤/脱嘧啶核酸内切酶 (APE1) 在 DNA 修复、转录调控和基因表达中起关键作用,是单金属依赖性核酸酶的典型例子。尽管 Mg 2+是天然金属辅因子,但 APE1 在多种金属存在下仍保持催化活性,随着 Mg 2+ > Mn 2+ > Ni 2+速率降低> Zn 2+,而 Ca 2+则完全废除了活性。目前的工作使用量子力学-分子力学技术来绘制在这些金属存在下 APE1 促进的磷酸二酯键水解。沿反应路径的固定点的结构差异揭示了允许 APE1 对各种金属保持催化活性的几个因素之间的相互作用,势垒高度的趋势与实验报告的 APE1 催化活性相关。相反,Ca 2+显着改变金属配位和活性位点几何形状,从而完全抑制催化作用。因此,我们的工作为有争议的单金属介导的磷酸二酯键断裂提供了支持,并澄清了有关金属和金属特性在这一重要反应中的作用的不确定性。这些信息是未来医学和生物技术应用的关键,包括疾病诊断和治疗以及蛋白质工程。
更新日期:2022-11-21
中文翻译:

单金属介导的磷酸二酯键断裂的金属依赖性:多方面人类酶的 QM/MM 研究
核酸酶使用一系列金属辅因子催化核酸中磷酸二酯键的裂解。尽管人们普遍认为许多核酸酶依赖于两种金属离子,但单金属介导的途径仍有争议。此外,单金属介导的核酸酶在许多不同金属存在的情况下保持活性,但这种广泛的金属特异性的潜在原因尚不清楚。人类脱嘌呤/脱嘧啶核酸内切酶 (APE1) 在 DNA 修复、转录调控和基因表达中起关键作用,是单金属依赖性核酸酶的典型例子。尽管 Mg 2+是天然金属辅因子,但 APE1 在多种金属存在下仍保持催化活性,随着 Mg 2+ > Mn 2+ > Ni 2+速率降低> Zn 2+,而 Ca 2+则完全废除了活性。目前的工作使用量子力学-分子力学技术来绘制在这些金属存在下 APE1 促进的磷酸二酯键水解。沿反应路径的固定点的结构差异揭示了允许 APE1 对各种金属保持催化活性的几个因素之间的相互作用,势垒高度的趋势与实验报告的 APE1 催化活性相关。相反,Ca 2+显着改变金属配位和活性位点几何形状,从而完全抑制催化作用。因此,我们的工作为有争议的单金属介导的磷酸二酯键断裂提供了支持,并澄清了有关金属和金属特性在这一重要反应中的作用的不确定性。这些信息是未来医学和生物技术应用的关键,包括疾病诊断和治疗以及蛋白质工程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号