当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of methane hydrate at ambient temperature with ultra-rapid formation and high gas storage capacity
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-11-21 , DOI: 10.1039/d2ee01968j Ye Zhang 1 , Jie Zhao 1, 2, 3 , Gaurav Bhattacharjee 1 , Huanzhi Xu 1 , Mingjun Yang 3 , Rajnish Kumar 4 , Praveen Linga 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-11-21 , DOI: 10.1039/d2ee01968j Ye Zhang 1 , Jie Zhao 1, 2, 3 , Gaurav Bhattacharjee 1 , Huanzhi Xu 1 , Mingjun Yang 3 , Rajnish Kumar 4 , Praveen Linga 1
Affiliation
|
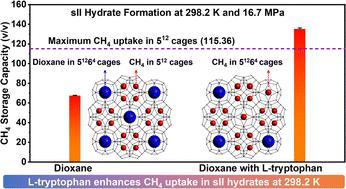
With the emergence of natural gas (NG) as a crucial transition fuel, NG storage techniques have also become essential components of nations’ energy resilience portfolios. The Solidified Natural Gas (SNG) technology is one emerging technique that promises safe, long-term NG storage under moderate pressure and temperature (P–T) conditions. Herein we investigate 1,3-dioxane (dioxane), a low-toxicity chemical additive, as a potential dual-function (thermodynamic and kinetic) promoter for the SNG technology. By enabling mixed methane (sII) hydrate formation, dioxane significantly moderates the P–T conditions required for SNG synthesis. At 283.2 K temperature and 7.2 MPa initial pressure, mixed CH4/dioxane (5.56 mol%) sII hydrate growth under unstirred conditions reaches 90% completion within 15 min. At ambient temperature (298.2 K) and an initial pressure of 16.7 MPa, a breakthrough volumetric methane storage capacity of 135.13 (±1.08) v/v (volume of gas at STP/volume of hydrate) is achieved, wherein methane molecules occupy about 34% of the sII-large (51264) cages in addition to all of the sII-small (512) cages demonstrating tunability of methane in the large cages. Finally, a mixed CH4/dioxane hydrate pellet, stored in a tightly sealed container under near atmospheric pressure of 135.2 kPa (gauge pressure) and a moderate average temperature of 268.3 (±0.2) K for 120 days, exhibits excellent stability throughout the duration of storage. The present study demonstrates that the gas storage capacity of methane in sII hydrate can be tuned to exceed the acknowledged limit of about 115.36 v/v and that sII hydrates can be readily and rapidly synthesized at room temperature, aiding the development of an environmentally and commercially viable SNG technology.
中文翻译:

常温下合成甲烷水合物,具有超快速形成和高储气能力
随着天然气 (NG) 作为重要的过渡燃料的出现,天然气储存技术也已成为各国能源弹性组合的重要组成部分。凝固天然气 (SNG) 技术是一项新兴技术,可在中等压力和温度 ( P – T ) 条件下实现安全、长期的天然气储存。在此,我们研究了 1,3-二恶烷 (dioxane),一种低毒性化学添加剂,作为 SNG 技术的潜在双功能(热力学和动力学)促进剂。通过促进混合甲烷 (sII) 水合物的形成,二恶烷显着缓和了SNG 合成所需的P – T条件。在 283.2 K 温度和 7.2 MPa 初始压力下,混合 CH 4/二恶烷 (5.56 mol%) sII 水合物在未搅拌条件下的生长在 15 分钟内达到 90%。在环境温度(298.2 K)和初始压力 16.7 MPa 下,实现了 135.13(±1.08)v/v(STP 气体体积/水合物体积)的甲烷突破体积存储容量,其中甲烷分子占据约 34除了所有 sII 小型 (5 12 ) 笼子之外,sII 大型 (5 12 6 4 ) 笼子的百分比表明大型笼子中甲烷的可调性。最后,混合 CH 4/二恶烷水合物颗粒在 135.2 kPa(表压)接近大气压力和 268.3 (±0.2) K 适中平均温度下储存在密闭容器中 120 天,在整个储存期间表现出出色的稳定性。本研究表明,甲烷在 sII 水合物中的储气能力可以调整到超过公认的约 115.36 v/v 的极限,并且 sII 水合物可以在室温下容易快速地合成,有助于开发环境和商业可行的 SNG 技术。
更新日期:2022-11-22
中文翻译:

常温下合成甲烷水合物,具有超快速形成和高储气能力
随着天然气 (NG) 作为重要的过渡燃料的出现,天然气储存技术也已成为各国能源弹性组合的重要组成部分。凝固天然气 (SNG) 技术是一项新兴技术,可在中等压力和温度 ( P – T ) 条件下实现安全、长期的天然气储存。在此,我们研究了 1,3-二恶烷 (dioxane),一种低毒性化学添加剂,作为 SNG 技术的潜在双功能(热力学和动力学)促进剂。通过促进混合甲烷 (sII) 水合物的形成,二恶烷显着缓和了SNG 合成所需的P – T条件。在 283.2 K 温度和 7.2 MPa 初始压力下,混合 CH 4/二恶烷 (5.56 mol%) sII 水合物在未搅拌条件下的生长在 15 分钟内达到 90%。在环境温度(298.2 K)和初始压力 16.7 MPa 下,实现了 135.13(±1.08)v/v(STP 气体体积/水合物体积)的甲烷突破体积存储容量,其中甲烷分子占据约 34除了所有 sII 小型 (5 12 ) 笼子之外,sII 大型 (5 12 6 4 ) 笼子的百分比表明大型笼子中甲烷的可调性。最后,混合 CH 4/二恶烷水合物颗粒在 135.2 kPa(表压)接近大气压力和 268.3 (±0.2) K 适中平均温度下储存在密闭容器中 120 天,在整个储存期间表现出出色的稳定性。本研究表明,甲烷在 sII 水合物中的储气能力可以调整到超过公认的约 115.36 v/v 的极限,并且 sII 水合物可以在室温下容易快速地合成,有助于开发环境和商业可行的 SNG 技术。











































 京公网安备 11010802027423号
京公网安备 11010802027423号