当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulating the electrocatalytic activity of mononuclear nickel complexes toward water oxidation by tertiary amine group
Dalton Transactions ( IF 4 ) Pub Date : 2022-11-21 , DOI: 10.1039/d2dt03381j Xiaoli Chen 1 , Xuehong Liao 1 , Chang Dai 1 , Lihong Zhu 1 , Li Hong 1 , Xueli Yang 1 , Zhijun Ruan 1 , Xiangming Liang 2 , Junqi Lin 1
Dalton Transactions ( IF 4 ) Pub Date : 2022-11-21 , DOI: 10.1039/d2dt03381j Xiaoli Chen 1 , Xuehong Liao 1 , Chang Dai 1 , Lihong Zhu 1 , Li Hong 1 , Xueli Yang 1 , Zhijun Ruan 1 , Xiangming Liang 2 , Junqi Lin 1
Affiliation
|
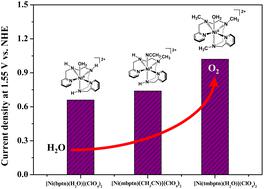
Water oxidation is the bottleneck of water splitting, which is a promising strategy for hydrogen production. Therefore, it is significant to develop efficient water oxidation catalysts. Herein, electrochemical water oxidation catalyzed by three nickel complexes, namely [Ni(bptn)(H2O)](ClO4)2 (1), [Ni(mbptn)(CH3CN)](ClO4)2 (2), and [Ni(tmbptn)(H2O)](ClO4)2 (3) (bptn = 1,9-bis(2-pyridyl)-2,5,8-triazanonane, mbptn = 5-methyl-1,9-bis(2-pyridyl)-2,5,8-triazanonane, and tmbptn = 1,9-bis(2-pyridyl)-2,5,8-triazanonane), is studied under near-neutral condition (pH 9.0). Meanwhile, the homogeneous catalytic behaviors of the three mononuclear nickel complexes were investigated and confirmed by scanning electron microscopy, energy dispersive spectrometry, X-ray photoelectron spectroscopy and electrochemical method. Complex 1 stabilized by a pentadentate ligand with three N–H fragments homogeneously catalyzes water oxidation to oxygen with the lowest onset overpotential. Complex 2 stabilized by a similar ligand with two N–H groups and one N-CH3 group exhibits relatively higher onset overpotential but higher catalytic current and turnover frequency. However, complex 3 with three N-CH3 coordination environment shows the highest onset overpotential and the highest catalytic current at higher potential. Comparison of catalytic behaviors and ligand structure of the three complexes reveals that the methyl group on the polypyridine amine ligand affects the water oxidation activity of the complexes obviously. The electronic effect of N-CH3 coordination environment leads to higher redox potential of the metal center and potential demand for water oxidation, while it leads to higher reaction activity of high-valent intermediates, which account for higher catalytic current and efficiency of water oxidation. This work reveals that electrocatalytic water oxidation performance of nickel complexes can be finely modulated by constructing suitable N-CH3 coordination.
中文翻译:

叔胺基团调控单核镍配合物对水氧化的电催化活性
水氧化是水分解的瓶颈,这是一种很有前途的制氢策略。因此,开发高效的水氧化催化剂具有重要意义。在此,电化学水氧化由三种镍配合物催化,即 [Ni(bptn)(H 2 O)](ClO 4 ) 2 ( 1 ),[Ni(mbptn)(CH 3 CN)](ClO 4 ) 2 ( 2 ), 和 [Ni(tmbptn)(H 2 O)](ClO 4 ) 2 ( 3) (bptn = 1,9-bis(2-pyridyl)-2,5,8-triazanonane, mbptn = 5-methyl-1,9-bis(2-pyridyl)-2,5,8-triazanonane, and tmbptn = 1,9-bis(2-pyridyl)-2,5,8-triazanonane),在近中性条件下 (pH 9.0) 进行研究。同时,通过扫描电镜、能谱、X射线光电子能谱和电化学方法研究并证实了三种单核镍配合物的均相催化行为。由具有三个 N-H 片段的五齿配体稳定的配合物 1 以最低的起始过电势均匀催化水氧化成氧气。由具有两个 N-H 基团和一个 N-CH 3的类似配体稳定的配合物2组表现出相对较高的起始过电位但较高的催化电流和转换频率。然而,具有三个N-CH 3配位环境的络合物3显示出最高的起始过电位和较高电位下的最高催化电流。比较三种配合物的催化行为和配体结构表明,聚吡啶胺配体上的甲基对配合物的水氧化活性有明显影响。N-CH 3的电子效应配位环境导致金属中心更高的氧化还原电位和水氧化的潜在需求,同时导致高价中间体的反应活性更高,从而导致更高的催化电流和水氧化效率。这项工作表明,可以通过构建合适的 N-CH 3配位来精细调节镍配合物的电催化水氧化性能。
更新日期:2022-11-21
中文翻译:

叔胺基团调控单核镍配合物对水氧化的电催化活性
水氧化是水分解的瓶颈,这是一种很有前途的制氢策略。因此,开发高效的水氧化催化剂具有重要意义。在此,电化学水氧化由三种镍配合物催化,即 [Ni(bptn)(H 2 O)](ClO 4 ) 2 ( 1 ),[Ni(mbptn)(CH 3 CN)](ClO 4 ) 2 ( 2 ), 和 [Ni(tmbptn)(H 2 O)](ClO 4 ) 2 ( 3) (bptn = 1,9-bis(2-pyridyl)-2,5,8-triazanonane, mbptn = 5-methyl-1,9-bis(2-pyridyl)-2,5,8-triazanonane, and tmbptn = 1,9-bis(2-pyridyl)-2,5,8-triazanonane),在近中性条件下 (pH 9.0) 进行研究。同时,通过扫描电镜、能谱、X射线光电子能谱和电化学方法研究并证实了三种单核镍配合物的均相催化行为。由具有三个 N-H 片段的五齿配体稳定的配合物 1 以最低的起始过电势均匀催化水氧化成氧气。由具有两个 N-H 基团和一个 N-CH 3的类似配体稳定的配合物2组表现出相对较高的起始过电位但较高的催化电流和转换频率。然而,具有三个N-CH 3配位环境的络合物3显示出最高的起始过电位和较高电位下的最高催化电流。比较三种配合物的催化行为和配体结构表明,聚吡啶胺配体上的甲基对配合物的水氧化活性有明显影响。N-CH 3的电子效应配位环境导致金属中心更高的氧化还原电位和水氧化的潜在需求,同时导致高价中间体的反应活性更高,从而导致更高的催化电流和水氧化效率。这项工作表明,可以通过构建合适的 N-CH 3配位来精细调节镍配合物的电催化水氧化性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号