当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding of human ACE2 and RBD of Omicron enhanced by unique interaction patterns among SARS-CoV-2 variants of concern
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-11-18 , DOI: 10.1002/jcc.27025 Seonghan Kim 1 , Yi Liu 1 , Matthew Ziarnik 1 , Sangjae Seo 2 , Yiwei Cao 3, 4, 5 , X Frank Zhang 1 , Wonpil Im 1, 3, 4, 5
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-11-18 , DOI: 10.1002/jcc.27025 Seonghan Kim 1 , Yi Liu 1 , Matthew Ziarnik 1 , Sangjae Seo 2 , Yiwei Cao 3, 4, 5 , X Frank Zhang 1 , Wonpil Im 1, 3, 4, 5
Affiliation
|
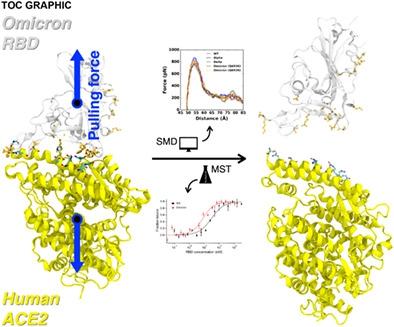
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing COVID-19, has continued to mutate and spread worldwide despite global vaccination efforts. In particular, the Omicron variant, first identified in South Africa in late November 2021, has become the dominant strain worldwide. Compared to the original strain identified in Wuhan, Omicron features 50 genetic mutations, with 15 mutations in the receptor-binding domain (RBD) of the spike protein, which binds to the human angiotensin-converting enzyme 2 (ACE2) receptor for viral entry. However, it is not completely understood how these mutations alter the interaction and binding strength between the Omicron RBD and ACE2. In this study, we used a combined steered molecular dynamics (SMD) simulation and experimental microscale thermophoresis (MST) approach to quantify the interaction between Omicron RBD and ACE2. We report that the Omicron brings an enhanced RBD-ACE2 interface through N501Y, Q498R, and T478K mutations; the changes further lead to unique interaction patterns, reminiscing the features of previously dominated variants, Alpha (N501Y) and Delta (L452R and T478K). Among the Q493K and Q493R, we report that Q493R shows stronger binding to ACE2 than Q493K due to increased interactions. Our MST data confirmed that the Omicron mutations in RBD are associated with a five-fold higher binding affinity to ACE2 compared to the RBD of the original strain. In conclusion, our results could help explain the Omicron variant's prevalence in human populations, as higher interaction forces or affinity for ACE2 likely promote greater viral binding and internalization, leading to increased infectivity.
中文翻译:

受关注的 SARS-CoV-2 变体之间独特的相互作用模式增强了人 ACE2 和 Omicron RBD 的结合
尽管全球都在努力开展疫苗接种工作,但引起 COVID-19 的严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 仍在全球范围内继续变异和传播。特别是 2021 年 11 月下旬在南非首次发现的 Omicron 变种,现已成为全球的主导菌株。与在武汉发现的原始毒株相比,Omicron 具有 50 个基因突变,其中刺突蛋白的受体结合域 (RBD) 有 15 个突变,该蛋白与人类血管紧张素转换酶 2 (ACE2) 受体结合,用于病毒进入。然而,尚不完全了解这些突变如何改变 Omicron RBD 和 ACE2 之间的相互作用和结合强度。在这项研究中,我们使用了组合的引导分子动力学 (SMD) 模拟和实验微尺度热泳 (MST) 方法来量化 Omicron RBD 和 ACE2 之间的相互作用。我们报告说,Omicron 通过 N501Y、Q498R 和 T478K 突变带来了增强的 RBD-ACE2 接口;这些变化进一步导致了独特的交互模式,让人想起以前占主导地位的变体 Alpha (N501Y) 和 Delta (L452R 和 T478K) 的特征。在 Q493K 和 Q493R 中,我们报告由于相互作用增加,Q493R 与 ACE2 的结合比 Q493K 更强。我们的 MST 数据证实,与原始菌株的 RBD 相比,RBD 中的 Omicron 突变与 ACE2 的结合亲和力高出五倍。总之,我们的结果可能有助于解释 Omicron 变体在人群中的流行,因为 ACE2 的相互作用力或亲和力越高,可能会促进更大的病毒结合和内化,从而导致传染性增加。
更新日期:2022-11-18
中文翻译:

受关注的 SARS-CoV-2 变体之间独特的相互作用模式增强了人 ACE2 和 Omicron RBD 的结合
尽管全球都在努力开展疫苗接种工作,但引起 COVID-19 的严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 仍在全球范围内继续变异和传播。特别是 2021 年 11 月下旬在南非首次发现的 Omicron 变种,现已成为全球的主导菌株。与在武汉发现的原始毒株相比,Omicron 具有 50 个基因突变,其中刺突蛋白的受体结合域 (RBD) 有 15 个突变,该蛋白与人类血管紧张素转换酶 2 (ACE2) 受体结合,用于病毒进入。然而,尚不完全了解这些突变如何改变 Omicron RBD 和 ACE2 之间的相互作用和结合强度。在这项研究中,我们使用了组合的引导分子动力学 (SMD) 模拟和实验微尺度热泳 (MST) 方法来量化 Omicron RBD 和 ACE2 之间的相互作用。我们报告说,Omicron 通过 N501Y、Q498R 和 T478K 突变带来了增强的 RBD-ACE2 接口;这些变化进一步导致了独特的交互模式,让人想起以前占主导地位的变体 Alpha (N501Y) 和 Delta (L452R 和 T478K) 的特征。在 Q493K 和 Q493R 中,我们报告由于相互作用增加,Q493R 与 ACE2 的结合比 Q493K 更强。我们的 MST 数据证实,与原始菌株的 RBD 相比,RBD 中的 Omicron 突变与 ACE2 的结合亲和力高出五倍。总之,我们的结果可能有助于解释 Omicron 变体在人群中的流行,因为 ACE2 的相互作用力或亲和力越高,可能会促进更大的病毒结合和内化,从而导致传染性增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号