当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Counteracting the Activity-Diastereoselectivity Trade-Off of l-Threonine Aldolase by Regulating the Proton Transfer Microenvironment
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-19 , DOI: 10.1002/adsc.202201006 Sai Fang 1 , Haoran Yu 1 , Lanxin Xiao 1 , Zhe Wang 1 , Yixuan Lei 1 , Gang Xu 2 , Li-Rong Yang 2 , Wenlong Zheng 1 , Jian-Ping Wu 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-19 , DOI: 10.1002/adsc.202201006 Sai Fang 1 , Haoran Yu 1 , Lanxin Xiao 1 , Zhe Wang 1 , Yixuan Lei 1 , Gang Xu 2 , Li-Rong Yang 2 , Wenlong Zheng 1 , Jian-Ping Wu 2
Affiliation
|
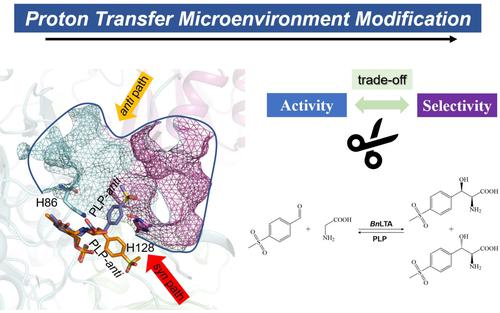
l-threonine aldolase (LTA) is a vital tool for the production of β-hydroxy-α-amino acids, important pharmaceutical intermediates with two chiral centres. However, the trade-off between activity and diastereoselectivity seriously hinders the application of LTA. Here, microenvironment of the proton transfer was regulated to improve the enzyme activity while avoiding the loss of diastereoselectivity. A combinatorial active-site saturation test (CAST) strategy was applied to engineer the microenvironment of the key histidines H86 and H128 involved in proton transfer. Except for the amino acid residues tunning diastereoselectivity, a total of 18 (9+9) residues lining around H86 and H128 were investigated. As a result, two variants, RS1-T92V and RS1-E123R, were obtained with specific activity from 9.61 U/mg to 11.24 U/mg and 14.41 U/mg, respectively. By combinatorial mutagenesis, a double-point mutant RS1-VR (T92V/E123R) was obtained with specific activity reaching 18.65 U/mg that was two-fold of the original strain (RS1). Notably, the mutant RS1-VR remained a high de value of 94.21%. Molecular dynamics (MD) simulations provided insights into the mechanism of activity-diastereoselectivity trade-off. The improvement of microenvironment contributes to reduce the swing amplitude of the side chain of H86, resulting in the proton transfer more efficient. This work provides a strategy of regulating the proton transfer microenvironment for counteracting the trade-off between activity and diastereoselectivity in protein engineering.
中文翻译:

通过调节质子转移微环境抵消 l-苏氨酸醛缩酶的活性-非对映选择性权衡
l -苏氨酸醛缩酶 ( L TA) 是生产β -羟基- α的重要工具-氨基酸,具有两个手性中心的重要医药中间体。然而,活性和非对映选择性之间的权衡严重阻碍了LTA的应用。在这里,调节质子转移的微环境以提高酶活性,同时避免非对映选择性的损失。应用组合活性位点饱和测试 (CAST) 策略来设计参与质子转移的关键组氨酸 H86 和 H128 的微环境。除了调节非对映选择性的氨基酸残基外,还研究了排列在 H86 和 H128 周围的总共 18 (9+9) 个残基。结果,获得了两种变体,RS1-T92V 和 RS1-E123R,比活性分别为 9.61 U/mg 至 11.24 U/mg 和 14.41 U/mg。通过组合诱变,获得双点突变株RS1-VR(T92V/E123R),比活性达到18.65 U/mg,是原株(RS1)的2倍。值得注意的是,突变体 RS1-VR 保持高de值94.21%。分子动力学 (MD) 模拟提供了对活性-非对映选择性权衡机制的见解。微环境的改善有助于降低H86侧链的摆动幅度,从而提高质子转移效率。这项工作提供了一种调节质子转移微环境的策略,以抵消蛋白质工程中活性和非对映选择性之间的权衡。
更新日期:2022-11-19
中文翻译:

通过调节质子转移微环境抵消 l-苏氨酸醛缩酶的活性-非对映选择性权衡
l -苏氨酸醛缩酶 ( L TA) 是生产β -羟基- α的重要工具-氨基酸,具有两个手性中心的重要医药中间体。然而,活性和非对映选择性之间的权衡严重阻碍了LTA的应用。在这里,调节质子转移的微环境以提高酶活性,同时避免非对映选择性的损失。应用组合活性位点饱和测试 (CAST) 策略来设计参与质子转移的关键组氨酸 H86 和 H128 的微环境。除了调节非对映选择性的氨基酸残基外,还研究了排列在 H86 和 H128 周围的总共 18 (9+9) 个残基。结果,获得了两种变体,RS1-T92V 和 RS1-E123R,比活性分别为 9.61 U/mg 至 11.24 U/mg 和 14.41 U/mg。通过组合诱变,获得双点突变株RS1-VR(T92V/E123R),比活性达到18.65 U/mg,是原株(RS1)的2倍。值得注意的是,突变体 RS1-VR 保持高de值94.21%。分子动力学 (MD) 模拟提供了对活性-非对映选择性权衡机制的见解。微环境的改善有助于降低H86侧链的摆动幅度,从而提高质子转移效率。这项工作提供了一种调节质子转移微环境的策略,以抵消蛋白质工程中活性和非对映选择性之间的权衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号