当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coordinating Anions “to the Rescue” of the Lithium Ion Mobility in Ternary Solid Polymer Electrolytes Plasticized With Ionic Liquids
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2022-11-18 , DOI: 10.1002/aenm.202202789 Jan‐Philipp Hoffknecht 1, 2 , Alina Wettstein 3 , Jaschar Atik 4 , Christian Krause 2 , Johannes Thienenkamp 4 , Gunther Brunklaus 4 , Martin Winter 2, 4 , Diddo Diddens 4 , Andreas Heuer 3, 4 , Elie Paillard 4, 5
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2022-11-18 , DOI: 10.1002/aenm.202202789 Jan‐Philipp Hoffknecht 1, 2 , Alina Wettstein 3 , Jaschar Atik 4 , Christian Krause 2 , Johannes Thienenkamp 4 , Gunther Brunklaus 4 , Martin Winter 2, 4 , Diddo Diddens 4 , Andreas Heuer 3, 4 , Elie Paillard 4, 5
Affiliation
|
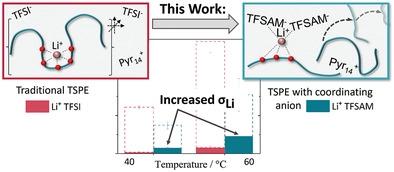
Lithium salts with low coordinating anions such as bis(trifluoromethanesulfonyl)imide (TFSI) have been the state-of-the-art for polyethylene oxide (PEO)-based “dry” polymer electrolytes for 3 decades. Plasticizing PEO with TFSI-based ionic liquids (ILs) to form ternary solid polymer electrolytes (TSPEs) increases conductivity and Li+ diffusivity. However, the Li+ transport mechanism is unaffected compared to their “dry” counterparts and is essentially coupled to the dynamics of the polymer host matrix, which limits Li+ transport improvement. Thus, a paradigm shift is hereby suggested: the utilization of more coordinating anions such as trifluoromethanesulfonyl-N-cyanoamide (TFSAM), able to compete with PEO for Li+ solvation, to accelerate the Li+ transport and reach a higher Li+ transference number. The Li–TFSAM interaction in binary and ternary TFSAM-based electrolytes is probed by experimental methods and discussed in the context of recent computational results. In PEO-based TSPEs, TFSAM drastically accelerates the Li+ transport (increases Li+ transference number by a factor 6 and the Li+ conductivity by 2–3) and computer simulations reveal that lithium dynamics are effectively re-coupled from polymer to anion dynamics. Last, this concept of coordinating anions in TSPEs is successfully applied in LFP||Li metal cells leading to enhanced capacity retention (86% after 300 cycles) and an improved rate performance at 2C.
中文翻译:

配位阴离子“拯救”用离子液体增塑的三元固体聚合物电解质中的锂离子迁移率
具有低配位阴离子的锂盐,例如双(三氟甲磺酰基)亚胺 (TFSI),3 年来一直是聚环氧乙烷 (PEO) 基“干”聚合物电解质的最先进技术。使用基于 TFSI 的离子液体 (IL) 增塑 PEO 以形成三元固体聚合物电解质 (TSPE) 可提高电导率和 Li +扩散率。然而,与它们的“干”对应物相比,Li +传输机制不受影响,并且本质上与聚合物主体基质的动力学耦合,这限制了 Li +传输的改进。因此,特此提出范式转变:利用更多的配位阴离子,例如三氟甲磺酰基-N-氰胺 (TFSAM),能够与 PEO 竞争 Li +溶剂化,加速 Li +传输并达到更高的 Li +转移数。二元和三元基于 TFSAM 的电解质中的 Li-TFSAM 相互作用通过实验方法进行了探索,并在最近的计算结果的背景下进行了讨论。在基于 PEO 的 TSPE 中,TFSAM 极大地加速了 Li +传输(将 Li +传输数增加了 6 倍,Li +电导率降低 2-3) 和计算机模拟表明锂动力学有效地从聚合物重新耦合到阴离子动力学。最后,这种在 TSPE 中配位阴离子的概念成功应用于 LFP||Li 金属电池,从而提高了容量保持率(300 次循环后为 86%)并提高了 2C 下的倍率性能。
更新日期:2022-11-18
中文翻译:

配位阴离子“拯救”用离子液体增塑的三元固体聚合物电解质中的锂离子迁移率
具有低配位阴离子的锂盐,例如双(三氟甲磺酰基)亚胺 (TFSI),3 年来一直是聚环氧乙烷 (PEO) 基“干”聚合物电解质的最先进技术。使用基于 TFSI 的离子液体 (IL) 增塑 PEO 以形成三元固体聚合物电解质 (TSPE) 可提高电导率和 Li +扩散率。然而,与它们的“干”对应物相比,Li +传输机制不受影响,并且本质上与聚合物主体基质的动力学耦合,这限制了 Li +传输的改进。因此,特此提出范式转变:利用更多的配位阴离子,例如三氟甲磺酰基-N-氰胺 (TFSAM),能够与 PEO 竞争 Li +溶剂化,加速 Li +传输并达到更高的 Li +转移数。二元和三元基于 TFSAM 的电解质中的 Li-TFSAM 相互作用通过实验方法进行了探索,并在最近的计算结果的背景下进行了讨论。在基于 PEO 的 TSPE 中,TFSAM 极大地加速了 Li +传输(将 Li +传输数增加了 6 倍,Li +电导率降低 2-3) 和计算机模拟表明锂动力学有效地从聚合物重新耦合到阴离子动力学。最后,这种在 TSPE 中配位阴离子的概念成功应用于 LFP||Li 金属电池,从而提高了容量保持率(300 次循环后为 86%)并提高了 2C 下的倍率性能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号