当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational investigation of the radical-mediated mechanism of formation of difluoro methyl oxindoles: Elucidation of the reaction selectivity and yields
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-11-18 , DOI: 10.1002/jcc.27031 Thanachon Somnarin 1, 2 , Pacharaporn Krawmanee 1 , Matthew Paul Gleeson 2 , Duangkamol Gleeson 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-11-18 , DOI: 10.1002/jcc.27031 Thanachon Somnarin 1, 2 , Pacharaporn Krawmanee 1 , Matthew Paul Gleeson 2 , Duangkamol Gleeson 1
Affiliation
|
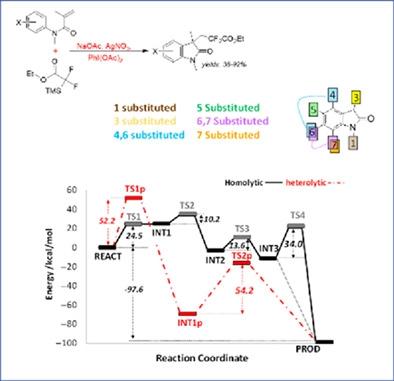
Oxindoles are an important class of heterocyclic alkaloids with demonstrated pharmacological activity at multiple biological targets. Preparation of new analogs through novel synthetic routes is therefore highly attractive. In this work, we report a computational study to investigate the synthesis of ethoxycarbonyldifluoromethylated oxindoles from N-arylmethacrylamides. The reaction tolerates a diverse range of acrylamides, shows yields ranging from approximately 38%–96%. We have applied density functional theory (DFT) to explore the reaction mechanism, kinetics and thermodynamics to gain further understanding. We demonstrate that a radical-based ring closure reaction is energetically more favorable than a heterolytic process, that the rate-determining step is the formation of the arylmethacrylamide radical, and that the product yields and selectivities are consistent with experiment. The results demonstrate that theoretical methods can prove useful to understand how such reaction and could be potentially employed to rapidly explore the reaction scope further.
中文翻译:

自由基介导的二氟甲基羟吲哚形成机制的计算研究:阐明反应选择性和产率
Oxindoles 是一类重要的杂环生物碱,在多个生物靶标上具有药理活性。因此,通过新的合成路线制备新的类似物极具吸引力。在这项工作中,我们报告了一项计算研究,以研究从N合成乙氧基羰基二氟甲基化羟吲哚-芳基甲基丙烯酰胺。该反应可耐受多种丙烯酰胺,显示产率范围约为 38%–96%。我们应用密度泛函理论 (DFT) 探索反应机理、动力学和热力学,以获得进一步的理解。我们证明基于自由基的闭环反应在能量上比异裂过程更有利,决定速率的步骤是芳基甲基丙烯酰胺自由基的形成,并且产物产率和选择性与实验一致。结果表明,理论方法可以证明有助于理解这种反应是如何发生的,并且可以潜在地用于进一步快速探索反应范围。
更新日期:2022-11-18
中文翻译:

自由基介导的二氟甲基羟吲哚形成机制的计算研究:阐明反应选择性和产率
Oxindoles 是一类重要的杂环生物碱,在多个生物靶标上具有药理活性。因此,通过新的合成路线制备新的类似物极具吸引力。在这项工作中,我们报告了一项计算研究,以研究从N合成乙氧基羰基二氟甲基化羟吲哚-芳基甲基丙烯酰胺。该反应可耐受多种丙烯酰胺,显示产率范围约为 38%–96%。我们应用密度泛函理论 (DFT) 探索反应机理、动力学和热力学,以获得进一步的理解。我们证明基于自由基的闭环反应在能量上比异裂过程更有利,决定速率的步骤是芳基甲基丙烯酰胺自由基的形成,并且产物产率和选择性与实验一致。结果表明,理论方法可以证明有助于理解这种反应是如何发生的,并且可以潜在地用于进一步快速探索反应范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号