当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multispectroscopic and Computational Investigations on the Binding Mechanism of Dicaffeoylquinic Acids with Ovalbumin
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-18 , DOI: 10.1021/acs.jcim.2c01011 Perumal Manivel 1 , Parthiban Marimuthu 2 , Sun Yu 1 , Xiumin Chen 1, 3, 4
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-18 , DOI: 10.1021/acs.jcim.2c01011 Perumal Manivel 1 , Parthiban Marimuthu 2 , Sun Yu 1 , Xiumin Chen 1, 3, 4
Affiliation
|
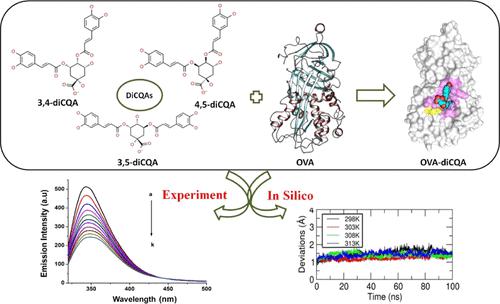
Recently, studies on the interactions between ovalbumin (OVA) and polyphenols have received a great deal of interest. This study explored the conformational changes and the interaction mechanism of the binding between OVA and chlorogenic acid (CGA) isomers such as 3,4-dicaffeoylquinic acids (3,4-diCQA), 4,5-dicaffeoylquinic acids (4,5-diCQA), and 3,5-dicaffeoylquinic acids (3,5-diCQA) using multispectroscopic and in silico analyses. The emission spectra show that the diCQAs caused strong quenching of OVA fluorescence under different temperatures through a static quenching mechanism with hydrogen bond (H-bond) and van der Waals (vdW) interactions. The values of binding constants (OVA–3,4-diCQA = 6.123 × 105, OVA–3,5-diCQA = 2.485 × 105, OVA–4,5-diCQA = 4.698 × 105 dm3 mol–1 at 298 K) suggested that diCQAs had a strong binding affinity toward OVA, among which OVA–3,4-diCQA exhibits higher binding constant. The results of UV–vis absorption and synchronous fluorescence indicated that the binding of all three diCQAs to OVA induced conformational and micro-environmental changes in the protein. The findings of molecular modeling further validate the significant role of vdW force and H-bond interactions in ensuring the stable binding of OVA–diCQA complexes. Temperature-dependent molecular dynamics simulation studies allow estimation of the individual components that contribute to the total bound free energy value, which allows evaluation of the nature of the interactions involved. This research can provide information for future investigations on food proteins’ physicochemical stability and CGA bioavailability in vitro or in vivo.
中文翻译:

二咖啡酰奎宁酸与卵清蛋白结合机制的多光谱和计算研究
最近,对卵清蛋白 (OVA) 和多酚之间相互作用的研究引起了极大的兴趣。本研究探讨了 OVA 与绿原酸 (CGA) 异构体如 3,4-二咖啡酰奎尼酸 (3,4-diCQA)、4,5-二咖啡酰奎尼酸 (4,5-diCQA) 结合的构象变化和相互作用机制), 和 3,5-二咖啡酰奎宁酸 (3,5-diCQA) 使用多光谱和计算机分析。发射光谱表明,diCQAs 通过氢键(H 键)和范德华 (vdW) 相互作用的静态猝灭机制,在不同温度下引起 OVA 荧光的强烈猝灭。结合常数值 (OVA–3,4-diCQA = 6.123 × 10 5 , OVA–3,5-diCQA = 2.485 × 10 5 , OVA–4,5-diCQA = 4.698 × 10 5分毫3摩尔–1at 298 K)表明diCQAs对OVA具有很强的结合亲和力,其中OVA-3,4-diCQA表现出更高的结合常数。紫外-可见吸收和同步荧光的结果表明,所有三种 diCQA 与 OVA 的结合诱导了蛋白质的构象和微环境变化。分子建模的结果进一步验证了 vdW 力和氢键相互作用在确保 OVA-diCQA 复合物稳定结合方面的重要作用。依赖于温度的分子动力学模拟研究可以估计对总结合自由能值有贡献的各个组分,从而可以评估所涉及的相互作用的性质。
更新日期:2022-11-18
中文翻译:

二咖啡酰奎宁酸与卵清蛋白结合机制的多光谱和计算研究
最近,对卵清蛋白 (OVA) 和多酚之间相互作用的研究引起了极大的兴趣。本研究探讨了 OVA 与绿原酸 (CGA) 异构体如 3,4-二咖啡酰奎尼酸 (3,4-diCQA)、4,5-二咖啡酰奎尼酸 (4,5-diCQA) 结合的构象变化和相互作用机制), 和 3,5-二咖啡酰奎宁酸 (3,5-diCQA) 使用多光谱和计算机分析。发射光谱表明,diCQAs 通过氢键(H 键)和范德华 (vdW) 相互作用的静态猝灭机制,在不同温度下引起 OVA 荧光的强烈猝灭。结合常数值 (OVA–3,4-diCQA = 6.123 × 10 5 , OVA–3,5-diCQA = 2.485 × 10 5 , OVA–4,5-diCQA = 4.698 × 10 5分毫3摩尔–1at 298 K)表明diCQAs对OVA具有很强的结合亲和力,其中OVA-3,4-diCQA表现出更高的结合常数。紫外-可见吸收和同步荧光的结果表明,所有三种 diCQA 与 OVA 的结合诱导了蛋白质的构象和微环境变化。分子建模的结果进一步验证了 vdW 力和氢键相互作用在确保 OVA-diCQA 复合物稳定结合方面的重要作用。依赖于温度的分子动力学模拟研究可以估计对总结合自由能值有贡献的各个组分,从而可以评估所涉及的相互作用的性质。









































 京公网安备 11010802027423号
京公网安备 11010802027423号