当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity of bi- and monometallic trifluoroacetates towards amorphous SiO2
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-11-18 , DOI: 10.1039/d2dt02822k Hashini N Munasinghe 1 , Marcos R Imer 1 , Regina G Szlag 1 , Leopoldo Suescun 2 , Federico A Rabuffetti 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-11-18 , DOI: 10.1039/d2dt02822k Hashini N Munasinghe 1 , Marcos R Imer 1 , Regina G Szlag 1 , Leopoldo Suescun 2 , Federico A Rabuffetti 1
Affiliation
|
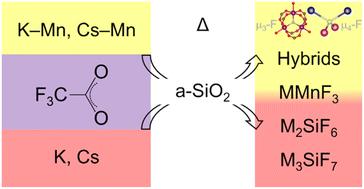
The reactivity of alkali–manganese(II) and alkali trifluoroacetates towards amorphous SiO2 (a-SiO2) was studied in the solid-state. K4Mn2(tfa)8, Cs3Mn2(tfa)7(tfaH), KH(tfa)2, and CsH(tfa)2 (tfa = CF3COO–) were thermally decomposed under vacuum in fused quartz tubes. Three new bimetallic fluorotrifluoroacetates of formulas K4Mn3(tfa)9F, Cs4Mn3(tfa)9F, and K2Mn(tfa)3F were discovered upon thermolysis at 175 °C. K4Mn3(tfa)9F and Cs4Mn3(tfa)9F feature a triangular-bridged metal cluster of formula [Mn3(μ3-F)(μ2-tfa)6(tfa)3]4−. In the case of K2Mn(tfa)3F, fluoride serves as an inverse coordination center for the tetrahedral metal cluster K2Mn2(μ4-F). Fluorotrifluoroacetates may be regarded as intermediates in the transformation of bimetallic trifluoroacetates to fluoroperovskites KMnF3, CsMnF3, and Cs2MnF4, which crystallized between 250 and 600 °C. Decomposition of these trifluoroacetates also yielded alkali hexafluorosilicates K2SiF6 and Cs2SiF6 as a result of the fluorination of fused quartz. The ability to fluorinate fused quartz was observed for monometallic alkali trifluoroacetates as well. Hexafluorosilicates and heptafluorosilicates K3SiF7 and Cs3SiF7 were obtained upon thermolysis of KH(tfa)2 and CsH(tfa)2 between 200 and 400 °C. This ability was exploited to synthesize fluorosilicates under air by simply reacting alkali trifluoroacetates with a-SiO2 powder.
中文翻译:

双金属和单金属三氟乙酸盐对无定形 SiO2 的反应性
研究了固态下碱金属锰( II )和碱金属三氟乙酸盐对无定形SiO 2 (a-SiO 2 )的反应性。 K 4 Mn 2 (tfa) 8 、Cs 3 Mn 2 (tfa) 7 (tfaH)、KH(tfa) 2和 CsH(tfa) 2 (tfa = CF 3 COO – ) 在熔融石英管中在真空下热分解。在 175 °C 热解时发现了三种新的双金属氟三氟乙酸盐,其化学式为 K 4 Mn 3 (tfa) 9 F、Cs 4 Mn 3 (tfa) 9 F 和 K 2 Mn(tfa) 3 F。 K 4 Mn 3 (tfa) 9 F 和 Cs 4 Mn 3 (tfa) 9 F 具有分子式为 [Mn 3 (μ 3 -F)(μ 2 -tfa) 6 (tfa) 3 ] 4的三角桥金属簇- .在K 2 Mn(tfa) 3 F的情况下,氟化物充当四面体金属簇K 2 Mn 2 (μ 4 -F)的逆配位中心。氟代三氟乙酸盐可被视为双金属三氟乙酸盐转化为氟钙钛矿KMnF 3 、CsMnF 3和Cs 2 MnF 4的中间体,其在250至600℃之间结晶。由于熔融石英的氟化,这些三氟乙酸盐的分解还产生碱金属六氟硅酸盐K 2 SiF 6和Cs 2 SiF 6 。还观察到单金属碱金属三氟乙酸盐具有氟化熔融石英的能力。 KH(tfa) 2和CsH(tfa) 2在200至400℃之间热解时获得六氟硅酸盐和七氟硅酸盐K 3 SiF 7和Cs 3 SiF 7 。利用这种能力,通过简单地将碱金属三氟乙酸盐与a-SiO 2粉末反应来在空气下合成氟硅酸盐。
更新日期:2022-11-18
中文翻译:

双金属和单金属三氟乙酸盐对无定形 SiO2 的反应性
研究了固态下碱金属锰( II )和碱金属三氟乙酸盐对无定形SiO 2 (a-SiO 2 )的反应性。 K 4 Mn 2 (tfa) 8 、Cs 3 Mn 2 (tfa) 7 (tfaH)、KH(tfa) 2和 CsH(tfa) 2 (tfa = CF 3 COO – ) 在熔融石英管中在真空下热分解。在 175 °C 热解时发现了三种新的双金属氟三氟乙酸盐,其化学式为 K 4 Mn 3 (tfa) 9 F、Cs 4 Mn 3 (tfa) 9 F 和 K 2 Mn(tfa) 3 F。 K 4 Mn 3 (tfa) 9 F 和 Cs 4 Mn 3 (tfa) 9 F 具有分子式为 [Mn 3 (μ 3 -F)(μ 2 -tfa) 6 (tfa) 3 ] 4的三角桥金属簇- .在K 2 Mn(tfa) 3 F的情况下,氟化物充当四面体金属簇K 2 Mn 2 (μ 4 -F)的逆配位中心。氟代三氟乙酸盐可被视为双金属三氟乙酸盐转化为氟钙钛矿KMnF 3 、CsMnF 3和Cs 2 MnF 4的中间体,其在250至600℃之间结晶。由于熔融石英的氟化,这些三氟乙酸盐的分解还产生碱金属六氟硅酸盐K 2 SiF 6和Cs 2 SiF 6 。还观察到单金属碱金属三氟乙酸盐具有氟化熔融石英的能力。 KH(tfa) 2和CsH(tfa) 2在200至400℃之间热解时获得六氟硅酸盐和七氟硅酸盐K 3 SiF 7和Cs 3 SiF 7 。利用这种能力,通过简单地将碱金属三氟乙酸盐与a-SiO 2粉末反应来在空气下合成氟硅酸盐。











































 京公网安备 11010802027423号
京公网安备 11010802027423号