当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Beryllium bonding with noble gas atoms
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-11-17 , DOI: 10.1002/jcc.27028 Lakhya Jyoti Mazumder 1 , Rohan Sharma 1 , Farnaz Yashmin 1 , Pankaz Kumar Sharma 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-11-17 , DOI: 10.1002/jcc.27028 Lakhya Jyoti Mazumder 1 , Rohan Sharma 1 , Farnaz Yashmin 1 , Pankaz Kumar Sharma 1
Affiliation
|
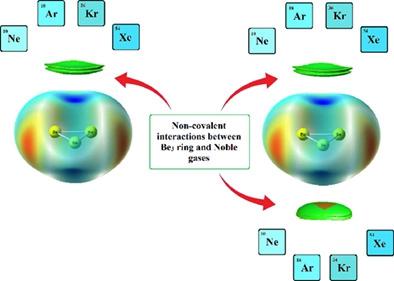
Quantum chemical calculations were carried out to investigate the nature of the bonding between a neutral Be3 ring and noble gas atom. Electronic structure calculation for these complexes was carried out at different computational levels in association with natural bond orbital, quantum theory of atoms in molecules, electron localization function, symmetry adapted perturbation theory, and molecular electrostatic potential surface analysis of Be3 complexes. The Be atoms in the Be3 moiety are chemically bonded to one another, with the BeBe bond dissociation energy being ~125 kJ mol−1. The Be3 ring interacts with the noble gases through non-covalent interactions. The binding energies of the noble gas atoms with the Be3 ring increases with increase in their atomic number. The non-covalent interaction index, density overlap region indicator and independent gradient model analyses reveal the presence of non-covalent inter-fragment interactions in the complexes. Energy decomposition analysis reveals that dispersion plays the major role towards stabilizing these systems.
中文翻译:

铍与惰性气体原子键合
进行了量子化学计算以研究中性 Be 3环和惰性气体原子之间键合的性质。结合自然键轨道、分子中原子的量子理论、电子局域化函数、对称适应微扰理论和Be 3配合物的分子静电势面分析,在不同的计算水平上对这些配合物进行了电子结构计算。Be 3部分中的 Be 原子相互化学键合,Be Be 键解离能为 ~125 kJ mol −1。Be 3环通过非共价相互作用与惰性气体相互作用。惰性气体原子与 Be 3环的结合能随着原子序数的增加而增加。非共价相互作用指数、密度重叠区域指标和独立梯度模型分析揭示了复合物中存在非共价片段间相互作用。能量分解分析表明,色散对稳定这些系统起着主要作用。
更新日期:2022-11-17
中文翻译:

铍与惰性气体原子键合
进行了量子化学计算以研究中性 Be 3环和惰性气体原子之间键合的性质。结合自然键轨道、分子中原子的量子理论、电子局域化函数、对称适应微扰理论和Be 3配合物的分子静电势面分析,在不同的计算水平上对这些配合物进行了电子结构计算。Be 3部分中的 Be 原子相互化学键合,Be Be 键解离能为 ~125 kJ mol −1。Be 3环通过非共价相互作用与惰性气体相互作用。惰性气体原子与 Be 3环的结合能随着原子序数的增加而增加。非共价相互作用指数、密度重叠区域指标和独立梯度模型分析揭示了复合物中存在非共价片段间相互作用。能量分解分析表明,色散对稳定这些系统起着主要作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号