当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
p38α Kinase Auto-Activation through Its Conformational Transition Induced by Tyr323 Phosphorylation
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-17 , DOI: 10.1021/acs.jcim.2c00236 Yongjian Zang 1 , He Wang 1 , Dongxiao Hao 1 , Ying Kang 1 , Jianwen Zhang 1 , Xuhua Li 1 , Lei Zhang 1 , Zhiwei Yang 1 , Shengli Zhang 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-11-17 , DOI: 10.1021/acs.jcim.2c00236 Yongjian Zang 1 , He Wang 1 , Dongxiao Hao 1 , Ying Kang 1 , Jianwen Zhang 1 , Xuhua Li 1 , Lei Zhang 1 , Zhiwei Yang 1 , Shengli Zhang 1
Affiliation
|
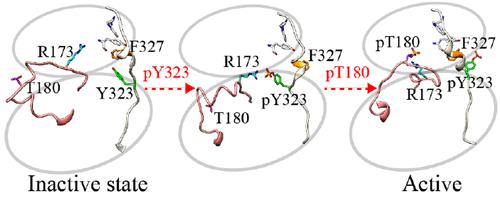
p38α is a key serine/threonine kinase that can enable atypical auto-activation through Zap70 phosphorylation and initiate T cell receptor signaling. The auto-activation plays an important role in autoimmune diseases. Although the classical activation mechanism of p38α has been studied in-depth, the atypical activation mechanism of Y323 phosphorylation-induced p38α auto-activation remains largely unexplained, especially the regulatory effects of phosphorylation on different sites (Y323 vs T180). From the X-ray experimental data, we identified the inactive and active states of p38α using principal component analysis. To understand the auto-activation process and the internal driving mechanism, a computational paradigm that couples the targeted molecular dynamics simulations, the String Method, and the umbrella sampling strategy were employed to generate the conformational landscape of p38α, including p38α T180–Y323, p38α T180–pY323, and p38α pT180–pY323 systems (pT180/pY323: phosphorylated T180/Y323). We explored that pY323 could change the conformational distribution and promote the conformational transition of p38α from the inactive state to the active state. Auto-activation of p38α is regulated by pY323 through destabilization of the hydrophobic core structure and aided by R173. This study will further explain the conformational transition of p38α induced by Y323 phosphorylation and provide insights into the universal molecular auto-activation mechanism of the p38 subfamily at the atomic level.
中文翻译:

p38α 激酶通过 Tyr323 磷酸化诱导的构象转变自动激活
p38α 是一种关键的丝氨酸/苏氨酸激酶,可通过 Zap70 磷酸化实现非典型自激活并启动 T 细胞受体信号传导。自身激活在自身免疫性疾病中起重要作用。尽管已经深入研究了 p38α 的经典激活机制,但 Y323 磷酸化诱导的 p38α 自激活的非典型激活机制在很大程度上仍未得到解释,尤其是磷酸化对不同位点的调节作用(Y323 与 T180)。从 X 射线实验数据中,我们使用主成分分析确定了 p38α 的非活性和活性状态。了解自动激活过程和内部驱动机制,耦合目标分子动力学模拟的计算范式,弦法,并采用伞形采样策略生成 p38α 的构象景观,包括 p38α T180–Y323、p38α T180–pY323 和 p38α pT180–pY323 系统(pT180/pY323:磷酸化 T180/Y323)。我们探索了 pY323 可以改变构象分布并促进 p38α 从非活性状态到活性状态的构象转变。p38α 的自动激活由 pY323 通过疏水核心结构的不稳定和 R173 的辅助来调节。本研究将进一步解释 Y323 磷酸化诱导的 p38α 构象转变,并在原子水平上提供对 p38 亚家族普遍分子自激活机制的见解。磷酸化的 T180/Y323)。我们探索了 pY323 可以改变构象分布并促进 p38α 从非活性状态到活性状态的构象转变。p38α 的自动激活由 pY323 通过疏水核心结构的不稳定和 R173 的辅助来调节。本研究将进一步解释 Y323 磷酸化诱导的 p38α 构象转变,并在原子水平上提供对 p38 亚家族普遍分子自激活机制的见解。磷酸化的 T180/Y323)。我们探索了 pY323 可以改变构象分布并促进 p38α 从非活性状态到活性状态的构象转变。p38α 的自动激活由 pY323 通过疏水核心结构的不稳定和 R173 的辅助来调节。本研究将进一步解释 Y323 磷酸化诱导的 p38α 构象转变,并在原子水平上提供对 p38 亚家族普遍分子自激活机制的见解。
更新日期:2022-11-17
中文翻译:

p38α 激酶通过 Tyr323 磷酸化诱导的构象转变自动激活
p38α 是一种关键的丝氨酸/苏氨酸激酶,可通过 Zap70 磷酸化实现非典型自激活并启动 T 细胞受体信号传导。自身激活在自身免疫性疾病中起重要作用。尽管已经深入研究了 p38α 的经典激活机制,但 Y323 磷酸化诱导的 p38α 自激活的非典型激活机制在很大程度上仍未得到解释,尤其是磷酸化对不同位点的调节作用(Y323 与 T180)。从 X 射线实验数据中,我们使用主成分分析确定了 p38α 的非活性和活性状态。了解自动激活过程和内部驱动机制,耦合目标分子动力学模拟的计算范式,弦法,并采用伞形采样策略生成 p38α 的构象景观,包括 p38α T180–Y323、p38α T180–pY323 和 p38α pT180–pY323 系统(pT180/pY323:磷酸化 T180/Y323)。我们探索了 pY323 可以改变构象分布并促进 p38α 从非活性状态到活性状态的构象转变。p38α 的自动激活由 pY323 通过疏水核心结构的不稳定和 R173 的辅助来调节。本研究将进一步解释 Y323 磷酸化诱导的 p38α 构象转变,并在原子水平上提供对 p38 亚家族普遍分子自激活机制的见解。磷酸化的 T180/Y323)。我们探索了 pY323 可以改变构象分布并促进 p38α 从非活性状态到活性状态的构象转变。p38α 的自动激活由 pY323 通过疏水核心结构的不稳定和 R173 的辅助来调节。本研究将进一步解释 Y323 磷酸化诱导的 p38α 构象转变,并在原子水平上提供对 p38 亚家族普遍分子自激活机制的见解。磷酸化的 T180/Y323)。我们探索了 pY323 可以改变构象分布并促进 p38α 从非活性状态到活性状态的构象转变。p38α 的自动激活由 pY323 通过疏水核心结构的不稳定和 R173 的辅助来调节。本研究将进一步解释 Y323 磷酸化诱导的 p38α 构象转变,并在原子水平上提供对 p38 亚家族普遍分子自激活机制的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号