当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size and Strain of Zinc Sulfide Nanoparticles Altered by Interaction with Organic Molecules
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-11-17 , DOI: 10.1021/acs.est.2c05268 Maureen Le Bars 1, 2, 3 , Clément Levard 1 , Samuel Legros 2, 3 , Vladimir Vidal 1 , Alejandro Fernandez-Martinez 4 , F Marc Michel 5 , Antoine Thill 6 , Benedicte Prelot 7 , Gabrielle Dublet-Adli 8 , Daniel Borschneck 1 , Jérôme Rose 1 , Emmanuel Doelsch 2, 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2022-11-17 , DOI: 10.1021/acs.est.2c05268 Maureen Le Bars 1, 2, 3 , Clément Levard 1 , Samuel Legros 2, 3 , Vladimir Vidal 1 , Alejandro Fernandez-Martinez 4 , F Marc Michel 5 , Antoine Thill 6 , Benedicte Prelot 7 , Gabrielle Dublet-Adli 8 , Daniel Borschneck 1 , Jérôme Rose 1 , Emmanuel Doelsch 2, 3
Affiliation
|
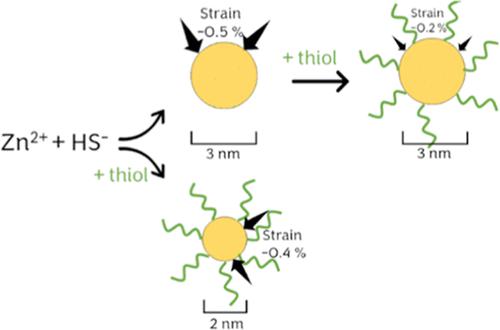
Nanosized zinc sulfides (nano-ZnS) have size-dependent and tunable physical and chemical properties that make them useful for a variety of technological applications. For example, structural changes, especially caused by strain, are pronounced in nano-ZnS < 5 nm in size, the size range typical of incidental nano-ZnS that form in the environment. Previous research has shown how natural organic matter impacts the physical properties of nano-ZnS but was mostly focused on their aggregation state. However, the specific organic molecules and the type of functional groups that are most important for controlling the nano-ZnS size and strain remain unclear. This study examined the size-dependent strain of nano-ZnS synthesized in the presence of serine, cysteine, glutathione, histidine, and acetate. Synchrotron total scattering pair distribution function analysis was used to determine the average crystallite size and strain. Among the different organic molecules tested, those containing a thiol group were shown to affect the particle size and size-induced strain most strongly when added during synthesis but significantly reduced the particle strain when added to as-formed nano-ZnS. The same effects are useful to understand the properties and behavior of natural nano-ZnS formed as products of microbial activity, for example, in reducing environments, or of incidental nano-ZnS formed in organic wastes.
中文翻译:

硫化锌纳米颗粒的尺寸和应变因与有机分子的相互作用而改变
纳米硫化锌 (nano-ZnS) 具有尺寸依赖性和可调谐的物理和化学特性,使其可用于各种技术应用。例如,结构变化,尤其是应变引起的结构变化,在尺寸 < 5 nm 的纳米 ZnS 中很明显,这是在环境中形成的偶然纳米 ZnS 的典型尺寸范围。先前的研究表明天然有机物如何影响纳米硫化锌的物理性质,但主要集中在它们的聚集状态上。然而,对于控制纳米 ZnS 尺寸和应变最重要的特定有机分子和官能团类型仍不清楚。本研究检测了在存在丝氨酸、半胱氨酸、谷胱甘肽、组氨酸和乙酸盐的情况下合成的纳米硫化锌的尺寸依赖性菌株。使用同步加速器总散射对分布函数分析来确定平均微晶尺寸和应变。在测试的不同有机分子中,含有硫醇基团的分子在合成过程中添加时显示对粒径和尺寸诱导应变的影响最强烈,但在添加到形成的纳米 ZnS 中时显着降低颗粒应变。同样的效应有助于理解作为微生物活动产物形成的天然纳米 ZnS 的特性和行为,例如,在还原环境中,或有机废物中偶然形成的纳米 ZnS。含有硫醇基团的那些在合成过程中添加时显示对粒径和尺寸诱导应变的影响最强烈,但在添加到形成的纳米 ZnS 中时显着降低了颗粒应变。同样的效应有助于理解作为微生物活动产物形成的天然纳米 ZnS 的特性和行为,例如,在还原环境中,或有机废物中偶然形成的纳米 ZnS。含有硫醇基团的那些在合成过程中添加时显示对粒径和尺寸诱导应变的影响最强烈,但在添加到形成的纳米 ZnS 中时显着降低了颗粒应变。同样的效应有助于理解作为微生物活动产物形成的天然纳米 ZnS 的特性和行为,例如,在还原环境中,或有机废物中偶然形成的纳米 ZnS。
更新日期:2022-11-17
中文翻译:

硫化锌纳米颗粒的尺寸和应变因与有机分子的相互作用而改变
纳米硫化锌 (nano-ZnS) 具有尺寸依赖性和可调谐的物理和化学特性,使其可用于各种技术应用。例如,结构变化,尤其是应变引起的结构变化,在尺寸 < 5 nm 的纳米 ZnS 中很明显,这是在环境中形成的偶然纳米 ZnS 的典型尺寸范围。先前的研究表明天然有机物如何影响纳米硫化锌的物理性质,但主要集中在它们的聚集状态上。然而,对于控制纳米 ZnS 尺寸和应变最重要的特定有机分子和官能团类型仍不清楚。本研究检测了在存在丝氨酸、半胱氨酸、谷胱甘肽、组氨酸和乙酸盐的情况下合成的纳米硫化锌的尺寸依赖性菌株。使用同步加速器总散射对分布函数分析来确定平均微晶尺寸和应变。在测试的不同有机分子中,含有硫醇基团的分子在合成过程中添加时显示对粒径和尺寸诱导应变的影响最强烈,但在添加到形成的纳米 ZnS 中时显着降低颗粒应变。同样的效应有助于理解作为微生物活动产物形成的天然纳米 ZnS 的特性和行为,例如,在还原环境中,或有机废物中偶然形成的纳米 ZnS。含有硫醇基团的那些在合成过程中添加时显示对粒径和尺寸诱导应变的影响最强烈,但在添加到形成的纳米 ZnS 中时显着降低了颗粒应变。同样的效应有助于理解作为微生物活动产物形成的天然纳米 ZnS 的特性和行为,例如,在还原环境中,或有机废物中偶然形成的纳米 ZnS。含有硫醇基团的那些在合成过程中添加时显示对粒径和尺寸诱导应变的影响最强烈,但在添加到形成的纳米 ZnS 中时显着降低了颗粒应变。同样的效应有助于理解作为微生物活动产物形成的天然纳米 ZnS 的特性和行为,例如,在还原环境中,或有机废物中偶然形成的纳米 ZnS。











































 京公网安备 11010802027423号
京公网安备 11010802027423号