当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suppressing Hydrogen Evolution in Aqueous Lithium-Ion Batteries with Double-Site Hydrogen Bonding
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-11-17 , DOI: 10.1021/acsenergylett.2c01993 Lixue Zhou 1, 2 , Songwei Tian 1 , Xiaofan Du 1 , Tingting Liu 1, 2 , Hao Zhang 3 , Jinning Zhang 1 , Sijia Hu 1 , Zheng Chen 1 , Jianjun Zhang 1 , Guanglei Cui 1, 4
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-11-17 , DOI: 10.1021/acsenergylett.2c01993 Lixue Zhou 1, 2 , Songwei Tian 1 , Xiaofan Du 1 , Tingting Liu 1, 2 , Hao Zhang 3 , Jinning Zhang 1 , Sijia Hu 1 , Zheng Chen 1 , Jianjun Zhang 1 , Guanglei Cui 1, 4
Affiliation
|
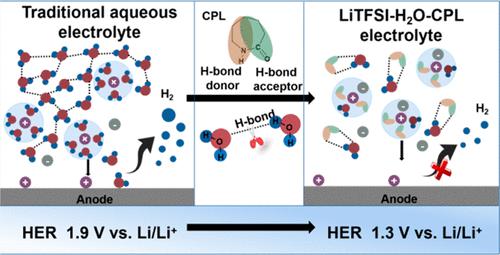
The recent concept of “molecular crowding agents” offering hydrogen bond (H-bond) accepting sites for free water molecules has alleviated parasitic hydrogen evolution in aqueous electrolytes. However, their cathodic limits are still not low enough to be compatible with the energy-dense Li4Ti5O12 anode (1.55 V vs Li+/Li). Inspired by nature’s choice of a peptide unit featuring an amide group in forming extensive H-bond networks with water, herein, we select caprolactam, an imide analogous to the amide group in a peptide, to reduce water activity via regulating the H-bond. The introduced caprolactam containing both an H-bond acceptor and donor effectively confines water molecules in a double-site anchoring configuration with strengthened H-bonding interactions and interrupts the original H-bonding among water molecules. This unique solution structure delays the onset potential of hydrogen evolution to 1.3 V vs Li+/Li, which enables the cycling of a Li4Ti5O12/LiMn2O4 full cell with an average Coulombic efficiency of 99.7% and 78% capacity retention after 350 cycles.
中文翻译:

通过双位点氢键抑制水系锂离子电池中的氢气释放
为自由水分子提供氢键(H 键)接受位点的“分子拥挤剂”的最新概念减轻了水性电解质中的寄生氢析出。然而,它们的阴极极限仍然不够低,无法与能量密集的 Li 4 Ti 5 O 12阳极兼容(1.55 V vs Li +/李)。受自然选择具有酰胺基团的肽单元与水形成广泛的氢键网络的启发,我们在此选择己内酰胺,一种类似于肽中酰胺基团的酰亚胺,通过调节氢键来降低水活度。引入的含有氢键受体和供体的己内酰胺有效地将水分子限制在双位点锚定构型中,增强了氢键相互作用,并中断了水分子之间的原始氢键。这种独特的溶液结构将氢析出的起始电位延迟到 1.3 V vs Li + /Li,这使得 Li 4 Ti 5 O 12 /LiMn 2 O 4的循环成为可能全电池在 350 次循环后平均库仑效率为 99.7%,容量保持率为 78%。
更新日期:2022-11-17
中文翻译:

通过双位点氢键抑制水系锂离子电池中的氢气释放
为自由水分子提供氢键(H 键)接受位点的“分子拥挤剂”的最新概念减轻了水性电解质中的寄生氢析出。然而,它们的阴极极限仍然不够低,无法与能量密集的 Li 4 Ti 5 O 12阳极兼容(1.55 V vs Li +/李)。受自然选择具有酰胺基团的肽单元与水形成广泛的氢键网络的启发,我们在此选择己内酰胺,一种类似于肽中酰胺基团的酰亚胺,通过调节氢键来降低水活度。引入的含有氢键受体和供体的己内酰胺有效地将水分子限制在双位点锚定构型中,增强了氢键相互作用,并中断了水分子之间的原始氢键。这种独特的溶液结构将氢析出的起始电位延迟到 1.3 V vs Li + /Li,这使得 Li 4 Ti 5 O 12 /LiMn 2 O 4的循环成为可能全电池在 350 次循环后平均库仑效率为 99.7%,容量保持率为 78%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号