当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Promoting electrocatalytic CO2 methanation using a molecular modifier on Cu surfaces
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-11-16 , DOI: 10.1039/d2ta07266a Cheng Wang 1 , Xiangdong Kong 1 , Junming Huang 1 , Yu Yang 2 , Han Zheng 1 , Huijuan Wang 1 , Suiyang Dai 1 , Shuzhen Zhang 2 , Yongxiang Liang 1 , Zhigang Geng 1 , Fengwang Li 2 , Jie Zeng 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-11-16 , DOI: 10.1039/d2ta07266a Cheng Wang 1 , Xiangdong Kong 1 , Junming Huang 1 , Yu Yang 2 , Han Zheng 1 , Huijuan Wang 1 , Suiyang Dai 1 , Shuzhen Zhang 2 , Yongxiang Liang 1 , Zhigang Geng 1 , Fengwang Li 2 , Jie Zeng 1
Affiliation
|
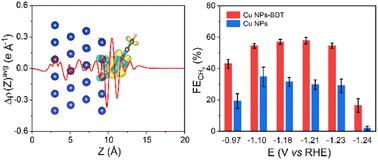
The electroreduction of CO2 to methane using renewable energy is a promising approach to achieving carbon neutrality. At commercially relevant current densities (>200 mA cm−2), methane selectivity is however below 50%. Herein, we reported a benzenethiol-modified Cu nanoparticle catalyst that achieved a methane faradaic efficiency of 54.5% at a partial current density of 383 mA cm−2, 1.9-fold higher than that of Cu nanoparticle controls. In situ vibrational spectroscopy and density functional theory calculations showed that the benzenethiol modulated the electronic structure of the Cu surface to enable a lowered coverage of *CO, favouring the formation of *CHO, a key intermediate embarking on the CH4 pathway, over the competing carbon–carbon coupling, the pathway towards multicarbons.
中文翻译:

在 Cu 表面使用分子改性剂促进电催化 CO2 甲烷化
使用可再生能源将 CO 2电还原为甲烷是实现碳中和的有前途的方法。然而,在商业相关的电流密度(>200 mA cm -2)下,甲烷选择性低于50%。在此,我们报道了一种苯硫醇改性的 Cu 纳米颗粒催化剂,其在 383 mA cm -2的部分电流密度下实现了 54.5% 的甲烷法拉第效率,比 Cu 纳米颗粒对照高 1.9 倍。原位振动光谱和密度泛函理论计算表明,苯硫酚调节了 Cu 表面的电子结构,降低了 *CO 的覆盖率,有利于 *CHO 的形成,*CHO 是 CH 4的关键中间体途径,通过竞争性碳 - 碳偶联,通往多碳的途径。
更新日期:2022-11-16
中文翻译:

在 Cu 表面使用分子改性剂促进电催化 CO2 甲烷化
使用可再生能源将 CO 2电还原为甲烷是实现碳中和的有前途的方法。然而,在商业相关的电流密度(>200 mA cm -2)下,甲烷选择性低于50%。在此,我们报道了一种苯硫醇改性的 Cu 纳米颗粒催化剂,其在 383 mA cm -2的部分电流密度下实现了 54.5% 的甲烷法拉第效率,比 Cu 纳米颗粒对照高 1.9 倍。原位振动光谱和密度泛函理论计算表明,苯硫酚调节了 Cu 表面的电子结构,降低了 *CO 的覆盖率,有利于 *CHO 的形成,*CHO 是 CH 4的关键中间体途径,通过竞争性碳 - 碳偶联,通往多碳的途径。









































 京公网安备 11010802027423号
京公网安备 11010802027423号