当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extended Solid-Solubility Limit in Layered Double Hydroxides: Tuning the Anion-Adsorption Selectivity
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.chemmater.2c02829 Tomohito Sudare 1 , Kenta Kawaguchi 2 , Kazuse Yamaguchi 2 , Kazuki Hirono 2 , Mongkol Tipplook 1 , Hideki Tanaka 1 , Fumitaka Hayashi 2 , Katsuya Teshima 1, 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-11-15 , DOI: 10.1021/acs.chemmater.2c02829 Tomohito Sudare 1 , Kenta Kawaguchi 2 , Kazuse Yamaguchi 2 , Kazuki Hirono 2 , Mongkol Tipplook 1 , Hideki Tanaka 1 , Fumitaka Hayashi 2 , Katsuya Teshima 1, 2
Affiliation
|
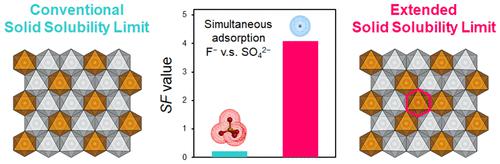
Unique atomic arrangements in nonthermodynamic solid solutions containing thermodynamically immiscible elements facilitate the introduction of new functionalities in various classes of materials, such as alloys, ceramics, and coordination polymers. Although high-temperature quenching has been widely used for synthesizing such materials with the aid of the surface effect and high configuration entropy, it is not applicable to materials that are unstable at high temperatures and contain only a few constituents. Thus, a new synthesis strategy is required. In this study, we demonstrated that a low-temperature topochemical reaction facilitates the extension of the conventional solid-solubility limit in layered double hydroxides (LDHs), a class of natural and synthetic lamellar compounds. Using a pristine layered oxide, Na[Ni1–xFex]O2 (0 < x < 1.0), the solid-solubility limit of LDHs ([Ni2+1–xFe3+x(OH)2]Cl–x·mH2O) could be extended from x = 0.33 to 0.59. Furthermore, compared to LDHs (x = 0.30), the obtained LDHs (x = 0.59) exhibited a 20-fold higher selectivity for fluoride ions than for sulfate ions. This selectivity possibly originated from a trivalent-cation clustering of Fe3+–Fe3+ neighbors in the LDH hydroxide layers; this caused a steric hindrance between sulfate and chloride ions in the interlayer space while preventing hindrance between fluoride and chloride ions. We expect that this study will guide the design of unexplored nonthermodynamic solid solutions.
中文翻译:

层状双氢氧化物中扩展的固溶度极限:调整阴离子吸附选择性
包含热力学不混溶元素的非热力学固溶体中独特的原子排列有助于在合金、陶瓷和配位聚合物等各类材料中引入新功能。尽管高温淬火借助表面效应和高构型熵已广泛用于合成此类材料,但不适用于高温不稳定且仅包含少量成分的材料。因此,需要一种新的合成策略。在这项研究中,我们证明了低温局部化学反应有助于扩展层状双氢氧化物 (LDH)(一类天然和合成的层状化合物)中的常规固溶度极限。使用原始的层状氧化物,Na[Ni 1–x Fe x ]O 2 (0 < x < 1.0), LDHs ([Ni 2+ 1– x Fe 3+ x (OH) 2 ]Cl – x · m H 2 O)的固溶度极限可以扩展从x = 0.33 到 0.59。此外,与 LDHs ( x = 0.30) 相比,获得的 LDHs ( x = 0.59) 对氟离子的选择性比对硫酸根离子的选择性高 20 倍。这种选择性可能源于 Fe 3+ –Fe 3+的三价阳离子簇LDH 氢氧化物层中的邻居;这在层间空间中造成了硫酸根离子和氯离子之间的空间位阻,同时防止了氟离子和氯离子之间的位阻。我们期望这项研究将指导未探索的非热力学固溶体的设计。
更新日期:2022-11-15
中文翻译:

层状双氢氧化物中扩展的固溶度极限:调整阴离子吸附选择性
包含热力学不混溶元素的非热力学固溶体中独特的原子排列有助于在合金、陶瓷和配位聚合物等各类材料中引入新功能。尽管高温淬火借助表面效应和高构型熵已广泛用于合成此类材料,但不适用于高温不稳定且仅包含少量成分的材料。因此,需要一种新的合成策略。在这项研究中,我们证明了低温局部化学反应有助于扩展层状双氢氧化物 (LDH)(一类天然和合成的层状化合物)中的常规固溶度极限。使用原始的层状氧化物,Na[Ni 1–x Fe x ]O 2 (0 < x < 1.0), LDHs ([Ni 2+ 1– x Fe 3+ x (OH) 2 ]Cl – x · m H 2 O)的固溶度极限可以扩展从x = 0.33 到 0.59。此外,与 LDHs ( x = 0.30) 相比,获得的 LDHs ( x = 0.59) 对氟离子的选择性比对硫酸根离子的选择性高 20 倍。这种选择性可能源于 Fe 3+ –Fe 3+的三价阳离子簇LDH 氢氧化物层中的邻居;这在层间空间中造成了硫酸根离子和氯离子之间的空间位阻,同时防止了氟离子和氯离子之间的位阻。我们期望这项研究将指导未探索的非热力学固溶体的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号