当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-catalyzed intramolecular C–H amination using aluminum nitrate as the oxidant
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-15 , DOI: 10.1039/d2qo01562e Kai Jia 1 , Yuan Xue 1 , Daoquan Tu 2 , Jun Luo 1 , Chao Jiang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-11-15 , DOI: 10.1039/d2qo01562e Kai Jia 1 , Yuan Xue 1 , Daoquan Tu 2 , Jun Luo 1 , Chao Jiang 1
Affiliation
|
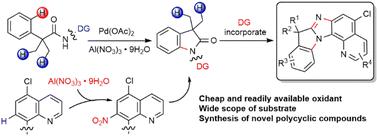
A palladium catalyzed intramolecular C(sp2)–H amination using a readily available aluminum nitrate (Al(NO3)3·9H2O) as the oxidant is reported. The C–H amination is promoted by in situ nitration of the quinoline directing group (DG). The oxindole products can be efficiently converted into novel polycyclic compounds (up to 7 fused rings) via a sequential reduction/cyclization involving the DG. Mechanistic studies indicate that nitration of the quinoline DG favors the following activation of γ-aryl C(sp2)–H bonds over β-methyl C(sp3)–H bonds.
中文翻译:

以硝酸铝为氧化剂的钯催化分子内C-H胺化反应
报道了使用容易获得的硝酸铝 (Al(NO 3 ) 3 ·9H 2 O) 作为氧化剂的钯催化分子内 C(sp 2 )–H 胺化反应。通过喹啉导向基团 (DG) 的原位硝化促进 C-H 胺化。通过涉及 DG 的顺序还原/环化,羟吲哚产物可以有效地转化为新型多环化合物(最多 7 个稠合环) 。机理研究表明,与 β-甲基 C(sp 3 )-H 键相比,喹啉 DG 的硝化有利于随后激活 γ-芳基 C(sp 2 ) -H 键。
更新日期:2022-11-15
中文翻译:

以硝酸铝为氧化剂的钯催化分子内C-H胺化反应
报道了使用容易获得的硝酸铝 (Al(NO 3 ) 3 ·9H 2 O) 作为氧化剂的钯催化分子内 C(sp 2 )–H 胺化反应。通过喹啉导向基团 (DG) 的原位硝化促进 C-H 胺化。通过涉及 DG 的顺序还原/环化,羟吲哚产物可以有效地转化为新型多环化合物(最多 7 个稠合环) 。机理研究表明,与 β-甲基 C(sp 3 )-H 键相比,喹啉 DG 的硝化有利于随后激活 γ-芳基 C(sp 2 ) -H 键。









































 京公网安备 11010802027423号
京公网安备 11010802027423号