当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cobalt-catalyzed asymmetric phospha-Michael reaction of diarylphosphine oxides for the synthesis of chiral organophosphorus compounds
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-14 , DOI: 10.1039/d2qo01483a Xu-Hui Yu 1 , Liang-Qiu Lu 1 , Zhi-Han Zhang 1 , De-Qing Shi 1 , Wen-Jing Xiao 1, 2
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2022-11-14 , DOI: 10.1039/d2qo01483a Xu-Hui Yu 1 , Liang-Qiu Lu 1 , Zhi-Han Zhang 1 , De-Qing Shi 1 , Wen-Jing Xiao 1, 2
Affiliation
|
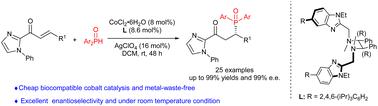
The asymmetric Michael addition of phosphorus nucleophiles to electron-deficient alkenes is one of the most direct and atom-economical methods to provide chiral organophosphorus compounds with high efficiency in recent years. Herein, we report a cobalt-catalyzed imidazolyl-directed asymmetric phospha-Michael-type reaction of diarylphosphine oxides with electron-deficient alkenes for synthesizing chiral organophosphorus compounds in moderate to good yields and good to excellent enantioselectivities (25 examples, up to 99% yield, and 99% ee). This protocol features broad substrate scope, good functional group tolerance, and mild conditions as well as avoids the release of massive metal wastes and the use of noble transition metal catalysts. The excellent enantioselectivity of the phospha-Michael reaction can be due to the adoption of a novel chiral N4-ligand. Furthermore, the DFT calculation indicates that the bulky 2,4,6-(i-Pr)3C6H2 group of the ligand induces large steric hindrance which blocks the nucleophilic attack from the Si-face.
中文翻译:

钴催化二芳基氧化膦的不对称磷迈克尔反应合成手性有机磷化合物
磷亲核试剂对缺电子烯烃的不对称迈克尔加成是近年来高效制备手性有机磷化合物最直接、原子经济的方法之一。在此,我们报道了钴催化的咪唑基导向的不对称磷酸-迈克尔型二芳基膦氧化物与缺电子烯烃的反应,以中等至良好的产率和良好至优异的对映选择性合成手性有机磷化合物(25 个例子,高达 99% 的收率) , 和 99% ee)。该方案具有广泛的底物范围、良好的官能团耐受性和温和的条件,并避免释放大量金属废物和使用贵金属过渡金属催化剂。磷酸-迈克尔反应出色的对映选择性可能是由于采用了一种新型手性 N4-配体。此外,DFT 计算表明体积庞大的 2,4,6-(i-Pr)配体的3 C 6 H 2基团诱导大的空间位阻,阻止来自Si面的亲核攻击。
更新日期:2022-11-14
中文翻译:

钴催化二芳基氧化膦的不对称磷迈克尔反应合成手性有机磷化合物
磷亲核试剂对缺电子烯烃的不对称迈克尔加成是近年来高效制备手性有机磷化合物最直接、原子经济的方法之一。在此,我们报道了钴催化的咪唑基导向的不对称磷酸-迈克尔型二芳基膦氧化物与缺电子烯烃的反应,以中等至良好的产率和良好至优异的对映选择性合成手性有机磷化合物(25 个例子,高达 99% 的收率) , 和 99% ee)。该方案具有广泛的底物范围、良好的官能团耐受性和温和的条件,并避免释放大量金属废物和使用贵金属过渡金属催化剂。磷酸-迈克尔反应出色的对映选择性可能是由于采用了一种新型手性 N4-配体。此外,DFT 计算表明体积庞大的 2,4,6-(i-Pr)配体的3 C 6 H 2基团诱导大的空间位阻,阻止来自Si面的亲核攻击。



























 京公网安备 11010802027423号
京公网安备 11010802027423号