当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Heteroannulation for the Synthesis of Quinoline-4-Carboxamides Bearing Axial Chirality
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-11 , DOI: 10.1002/adsc.202201043 Xiang Zhang 1 , Bo-Tao Chen 1 , Peng Gao 1 , Rui-Ping Song 1 , Fang Fang 1 , Dan-Dan Han 2 , You-Dong Shao 2 , Dao-Juan Cheng 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-11-11 , DOI: 10.1002/adsc.202201043 Xiang Zhang 1 , Bo-Tao Chen 1 , Peng Gao 1 , Rui-Ping Song 1 , Fang Fang 1 , Dan-Dan Han 2 , You-Dong Shao 2 , Dao-Juan Cheng 1
Affiliation
|
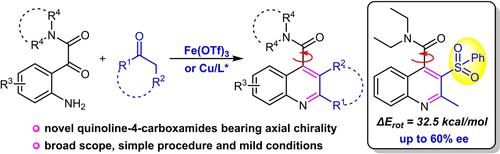
An iron(III) triflate catalyzed Friedländer-type heteroannulation of 2-(2-aminophenyl)-N,N-dialkyl-2-oxoamides with α-methylene carbonyl derivatives has been established, allowing facile access to a range of biologically interesting quinoline-containing tertiary amides in good yields. Investigations into the configurational stability of products led to the identification of a new type of stable axially chiral quinoline-4-carboxamide bearing a sterically demanding phenylsulfonyl C3-substituent. Subsequently, an enantioselective catalytic variant was attempted. The employment of copper(II) catalysis using a chiral bisoxazoline ligand was found to be capable of providing moderate enantiocontrol.
中文翻译:

催化杂环化合成具有轴向手性的喹啉-4-甲酰胺
已经建立了三氟甲磺酸铁 (III) 催化的 2-(2-氨基苯基)- N , N -二烷基-2-氧代酰胺与 α-亚甲基羰基衍生物的弗里德兰德型杂环化,从而可以轻松获得一系列具有生物学意义的喹啉-含有叔酰胺,收率高。对产品构型稳定性的研究导致鉴定出一种新型稳定的轴向手性喹啉-4-甲酰胺,带有空间要求严格的苯磺酰基 C3-取代基。随后,尝试了对映选择性催化变体。发现使用手性双恶唑啉配体的铜 (II) 催化能够提供适度的对映体控制。
更新日期:2022-11-11
中文翻译:

催化杂环化合成具有轴向手性的喹啉-4-甲酰胺
已经建立了三氟甲磺酸铁 (III) 催化的 2-(2-氨基苯基)- N , N -二烷基-2-氧代酰胺与 α-亚甲基羰基衍生物的弗里德兰德型杂环化,从而可以轻松获得一系列具有生物学意义的喹啉-含有叔酰胺,收率高。对产品构型稳定性的研究导致鉴定出一种新型稳定的轴向手性喹啉-4-甲酰胺,带有空间要求严格的苯磺酰基 C3-取代基。随后,尝试了对映选择性催化变体。发现使用手性双恶唑啉配体的铜 (II) 催化能够提供适度的对映体控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号